Pressure-retarded osmosis
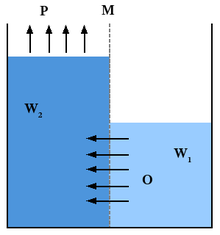
Pressure retarded osmosis (PRO) is a technique to separate a solvent (for example, fresh water) from a solution that is more concentrated (e.g. sea water) and also pressurized. A semipermeable membrane allows the solvent to pass to the concentrated solution side by osmosis.[1] The technique can be used to generate power from the salinity gradient energy resulting from the difference in the salt concentration between sea and river water. In PRO, the water potential between fresh water and sea water corresponds to a pressure of 26 bars. This pressure is equivalent to a column of water (hydraulic head) 270 meters high.[2] However, the optimal working pressure is only half of this, 11 to 15 bar.[3]
History
This method of generating power was invented by Prof. Sidney Loeb in 1973 at the Ben-Gurion University of the Negev, Beersheba, Israel.[4] [5] In 2014 researchers verified that 95% of a PRO system's theoretical power output can be produced with a membrane that is half (or less) the size needed for achieving 100%. Output is proportional to the salinity. Desalination yields very salty brine, while treated municipal wastewater has relatively little salt. Combining those streams could produce energy to power both facilities. However, powering an existing wastewater treatment plant by mixing treated wastewater with seawater could require a membrane area of 2.5 million square meters.[6]
To deal with these membrane requirements, scientists are working on rolled membranes [7] that will take up much less space.
Testing
The world's first osmotic plant with capacity of 10 kW was opened by Statkraft, a state-owned hydropower company, on 24 November 2009 in Tofte, Norway.[8] In January 2014 Statkraft terminated their osmosis pilot project [9] due to economic feasibility concerns.
It is estimated that each year 1600 TWh could be generated worldwide, and 12 TWh in Norway, sufficient to meet 10% of Norway's total demand for electricity.[10]
In Denmark, SaltPower is building the worlds first commercial osmitic power plant using high salinity brine. [11]
See also
- Electrodialysis reversal (EDR)
- Forward osmosis
- Green energy
- Osmotic power
- Osmotic pressure
- Renewable energy
- Reverse electrodialysis (RED)
- Reverse osmosis
- Semipermeable membrane
- Van 't Hoff factor
References
- ^ Helfer Fernanda, Lemckert Charles, Anissimov Yuri G (2014). "Osmotic power with Pressure Retarded Osmosis: Theory, performance and trends – A review". Journal of Membrane Science. 453: 337–358. doi:10.1016/j.memsci.2013.10.053. hdl:10072/61191.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ How does it work? Archived 2009-11-28 at the Wayback Machine - Statkraft
- ^ Osmoosivoimalan toiminta[permanent dead link] - Tekniikka & Talous (in Finnish)
- ^ Israel Patent Application 42658. (July 3, 1973) United States patent 3906250. (Erroneously shows Israel priority as 1974 instead of 1973).
- ^ Weintraub, Bob. "Sidney Loeb and the origins of pressure retareded osmosis". The Israel Chemist and Chemical Engineer, 2021.
- ^ Akst, Daniel (29 August 2014). "A New Kind of Power from Salt Water". Wall Street Journal.
- ^ "A New Kind of Power from Salt Water" Wall St. Journal, August 29–31, 2014 [1]
- ^ Wojciech Moskwa (2009-11-24). "World's First Osmotic Power Plant Opens". Reuters. Retrieved 2014-08-23.
- ^ "Is PRO economically feasible? Not according to Statkraft | ForwardOsmosisTech". 22 January 2014.
- ^ Statkraft to build world's first osmotic power plant Archived 2008-09-15 at the Wayback Machine
- ^ "SaltPower i Sønderborg har solgt sit første salt-kraftværk | SønderborgNYT".
Further reading
- Loeb S.; Norman R. S. (1975). "Osmotic Power Plants". Science. 189 (4203): 654–655. Bibcode:1975Sci...189..654L. doi:10.1126/science.189.4203.654. PMID 17838753.
- Loeb S. (1998). "Energy Production at the Dead Sea by Pressure-Retarded Osmosis: Challenge or Chimera?". Desalination. 120 (3): 247–262. doi:10.1016/S0011-9164(98)00222-7.
- Norman R. S. (1974). "Water Salination: A Source of Energy". Science. 186 (4161): 350–2. Bibcode:1974Sci...186..350N. doi:10.1126/science.186.4161.350. PMID 17839865. S2CID 8550368.
- Cath T. Y.; Childress A. E.; Elimelech M. (2006). "Forward osmosis: Principles, applications, and recent developments (Review)". Journal of Membrane Science. 281 (1–2): 70–87. doi:10.1016/j.memsci.2006.05.048.
- Loeb S. (1988). "Comments on the suitability of reverse osmosis membranes for energy recover by submarine osmotic power plants Desalination (Review)". Journal of Membrane Science. 68: 75–76. doi:10.1016/0011-9164(88)80044-4.
- Loeb S. (2002). "Large-scale power production by pressure-retarded osmosis, using river water and sea water passing through spiral modules desalination (Review)". Journal of Membrane Science. 143 (2): 115–122. doi:10.1016/S0011-9164(02)00233-3.
- Achilli A.; Cath T. Y.; Childress A. E. (2009). "Power generation with pressure retarded osmosis: an experimental and theoretical investigation". Journal of Membrane Science. 343 (1–2): 42–52. doi:10.1016/j.memsci.2009.07.006.

