Agarose gel electrophoresis: Difference between revisions
No edit summary |
m Reverted edits by 199.106.86.2 to last version by Teddks (HG) |
||
| Line 1: | Line 1: | ||
[[Image:DNAgel4wiki.png|200px|thumb|right|Digital image of 3 plasmid restriction digests run on a 1% w/v agarose gel, 3 Volts/cm, stained with ethidium bromide. The DNA size marker is a commercial 1 kbp ladder. The position of the wells and direction of DNA migration is noted.]] |
[[Image:DNAgel4wiki.png|200px|thumb|right|Digital image of 3 plasmid restriction digests run on a 1% w/v agarose gel, 3 Volts/cm, stained with ethidium bromide. The DNA size marker is a commercial 1 kbp ladder. The position of the wells and direction of DNA migration is noted.]] |
||
'''Agarose [[gel electrophoresis]]''' is a method used in [[biochemistry]] and [[molecular biology]] to separate [[DNA]], or [[RNA]] molecules by size. This is achieved by moving negatively charged nucleic acid molecules through an [[agarose]] matrix with an [[electric field]] ([[electrophoresis]]). Shorter molecules move faster and migrate farther than longer ones. |
'''Agarose [[gel electrophoresis]]''' is a method used in [[biochemistry]] and [[molecular biology]] to separate [[DNA]], or [[RNA]] molecules by size. This is achieved by moving negatively charged nucleic acid molecules through an [[agarose]] matrix with an [[electric field]] ([[electrophoresis]]). Shorter molecules move faster and migrate farther than longer ones.<ref>Sambrook J, Russel DW (2001). Molecular Cloning: A Laboratory Manual 3rd Ed. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY.</ref> |
||
==Applications== |
==Applications== |
||
| Line 7: | Line 7: | ||
*Analysis of [[PCR]] products, e.g. in molecular [[Preimplantation genetic diagnosis|genetic diagnosis]] or [[genetic fingerprinting]] |
*Analysis of [[PCR]] products, e.g. in molecular [[Preimplantation genetic diagnosis|genetic diagnosis]] or [[genetic fingerprinting]] |
||
*Separation of restricted genomic DNA prior to [[Southern blot|Southern transfer]], or of RNA prior to [[Northern Blot|Northern transfer]]. |
*Separation of restricted genomic DNA prior to [[Southern blot|Southern transfer]], or of RNA prior to [[Northern Blot|Northern transfer]]. |
||
The advantages are that the gel is easily poured, does not denature the samples. The samples can also be recovered. |
|||
The disadvantages are that gels can melt during electrophoresis, the buffer can become exhausted, and different forms of genetic material may run in unpredictable forms. |
|||
==Factors affecting migration== |
|||
The most important factor is the length of the DNA molecule, smaller molecules travel farther. But [[conformation]] of the DNA molecule is also a factor. To avoid this problem linear molecules are usually separated, usually DNA fragments from a [[restriction digest]], linear DNA [[PCR]] products, or RNAs. |
|||
Increasing the agarose concentration of a gel reduces the migration speed and enables separation of smaller DNA molecules. The higher the voltage, the faster the DNA moves. But voltage is limited by the fact that it heats and ultimately causes the gel to melt. High voltages also decrease the resolution (above about 5 to 8 V/cm). |
|||
Conformations of a DNA [[plasmid]] that has not been cut with a [[restriction enzyme]] will move with different speeds (slowest to fastest): nicked or open circular, linearised, or supercoiled plasmid. |
|||
==Visualisation: Ethidium Bromide (EtBr) and dyes== |
|||
The most common dye used to make DNA or RNA bands visible for agarose gel electrophoresis is [[ethidium bromide]], usually abbreviated as EtBr. It fluoresces under UV light when intercalated into DNA (or RNA). By running DNA through an EtBr-treated gel and visualizing it with UV light, any band containing more than ~20ng DNA becomes distinctly visible. EtBr is a known carcinogen, however, and safer alternatives are available. |
|||
[[SYBR Green|SYBR Green I]] is another dsDNA stain, produced by [[Invitrogen]]. It is more expensive, but 25 times more sensitive, and possibly safer than EtBr, though there is no data addressing its mutagenicity or toxicity in humans.<ref>[http://probes.invitrogen.com/media/pis/mp07567.pdf SYBR Green I Nucleic Acid Gel Stain<!-- Bot generated title -->]</ref> |
|||
[[SYBR Safe]] is a variant of SYBR Green that has been shown to have low enough levels of mutagenicity and toxicity to be deemed nonhazardous waste under U.S. Federal regulations.<ref name="probes.invitrogen.com">[http://probes.invitrogen.com/media/pis/mp33100.pdf SYBR Safe DNA Gel Stain<!-- Bot generated title -->]</ref> It has similar sensitivity levels to EtBr,<ref name="probes.invitrogen.com"/> but, like SYBR Green, is significantly more expensive. |
|||
Since EtBr stained DNA is not visible in natural light, scientists mix DNA with negatively charged '''loading buffers''' before adding the mixture to the gel. Loading buffers are useful because they are visible in natural light (as opposed to UV light for EtBr stained DNA), and they co-sediment with DNA (meaning they move at the same speed as DNA of a certain length). [[Xylene cyanol]] and [[Bromophenol blue]] are common loading buffers; they run about the same speed as DNA fragments that are 5000 bp and 300 bp in length respectively, but the precise position varies with percentage of the gel. Other less frequently used progress markers are [[Cresol Red]] and [[Orange G]] which run at about 125 bp and 50 bp. |
|||
==Percent agarose and resolution limits== |
|||
Agarose gel electrophoresis can be used for the separation of DNA fragments ranging from 50 [[base pair]] to several megabases (millions of bases) using specialized apparatus. The distance between DNA bands of a given length is determined by the percent agarose in the gel. In general lower concentrations of agarose are better for larger molecules because they result in greater separation between bands that are close in size. The disadvantage of higher concentrations is the long run times (sometimes days). Instead high percentage agarose gels should be run with a [[Pulsed field gel electrophoresis|pulsed field electrophoresis]] (PFE), or [[field inversion electrophoresis]]. |
|||
Most agarose gels are made with between 0.7% (good separation or resolution of large 5–10kb DNA fragments) and 2% (good resolution for small 0.2–1kb fragments) agarose dissolved in electrophoresis buffer. Some people go as high as 3% for separating very tiny fragments but a vertical [[polyacrylamide gel]] is more appropriate in this case. Low percentage gels are very weak and may break when you try to lift them. High percentage gels are often brittle and do not set evenly. 1% gels are common for many applications. |
|||
==Buffers== |
|||
There are a number of buffers used for agarose electrophoresis. The most common being: tris acetate [[EDTA]] (TAE), [[TBE buffer|Tris/Borate/EDTA]] (TBE) and [[Sodium borate]] (SB). TAE has the lowest buffering capacity but provides the best resolution for larger DNA. This means a lower voltage and more time, but a better product. SB is relatively new and is ineffective in resolving fragments larger than 5 kbp; However, with its low conductivity, a much higher voltage could be used (up to 35 V/cm), which means a shorter analysis time for routine electrophoresis. As low as one base pair size difference could be resolved in 3% agarose gel with an extremely low conductivity medium (1 mM Lithium borate).<ref>Brody JR, Calhoun ES, Gallmeier E, Creavalle TD, Kern SE (2004). Ultra-fast high-resolution agarose electrophoresis of DNA and RNA using low-molarity conductive media. Biotechniques. 37:598-602. [http://www.biotechniques.com/default.asp?page=article_archive&subsection=article_display&id=101200415&prevpage=article_archive]</ref> |
|||
==Analysis== |
|||
After electrophoresis the gel is illuminated with an [[ultraviolet]] lamp (usually by placing it on a light box, while using protective gear to limit exposure to ultraviolet radiation) to view the DNA bands. The [[ethidium bromide]] [[fluorescence|fluoresces]] reddish-orange in the presence of DNA. The DNA band can also be cut out of the gel, and can then be dissolved to retrieve the purified DNA. |
|||
The gel can then be photographed usually with a digital or polaroid camera. Although the stained nucleic acid fluoresces reddish-orange, images are usually shown in black and white (see figures). |
|||
Gel electrophoresis research often takes advantage of software-based image analysis tools, such as [[ImageJ]]. |
|||
{| class="wikitable" |
|||
|- |
|||
! 1 |
|||
! 2 |
|||
! 3 |
|||
|- |
|||
| [[Image:Agarosegel.jpg|200px|thumb|top| A 1% agarose 'slab' gel prior to UV illumination, behind a perspex UV shield. Only the marker dyes can be seen]] |
|||
| [[Image:AgarosegelUV.jpg|200px|thumb|top| The gel with UV illumination, the [[ethidium bromide]] stained DNA glows orange]] |
|||
| [[Image:Agarosegelphoto.jpg|200px|thumb|top| Digital photo of the gel. Lane 1. Commercial DNA Markers (1kbplus), Lane 2. empty, Lane 3. a [[PCR]] product of just over 500 bases, Lane 4. [[Restriction enzyme|Restriction]] digest showing the a similar fragment cut from a 4.5 kb [[plasmid]] vector]] |
|||
|- |
|||
|} |
|||
==Typical method== |
|||
===Materials=== |
|||
Typically 10-30 μl/sample of the DNA fragments to separate are obtained, as well as a mixture of DNA fragments (usually 10-20) of known size (after processing with DNA size markers either from a commercial source or prepared manually). |
|||
*[[Buffer solution]], usually [[TBE buffer]] or TAE 1.0x, pH 8.0 |
|||
*[[Agarose]] |
|||
An ultraviolet-fluorescent dye, [[ethidium bromide]], (5.25 mg/ml in H<sub>2</sub>O). The [[stock solution]] be careful handling this. |
|||
::Alternative dyes may be used, such as [[SYBR Green]]. |
|||
*[[Nitrile rubber]] gloves |
|||
::Latex gloves do not protect well from [[ethidium bromide]] |
|||
*A color marker dye containing a low [[molecular weight]] [[dye]] such as "[[bromophenol blue]]" (to enable tracking the progress of the electrophoresis) and glycerol (to make the DNA solution denser so it will sink into the wells of the gel). |
|||
*A gel rack |
|||
*A "comb" |
|||
*Power Supply |
|||
*UV lamp or UV lightbox or other method to visualize DNA in the gel |
|||
===Preparation=== |
|||
There are several methods for preparing gels. A common example is shown here. Other methods might differ in the buffering system used, the sample size to be loaded, the total volume of the gel (typically thickness is kept to a constant amount while length and breadth are varied as needed). Most agarose gels used in modern [[biochemistry]] and [[molecular biology]] are prepared and run horizontally. |
|||
#Make a 1% agarose solution in 100ml TAE, for typical DNA fragments (see figures). A solution of up to 2-4% can be used if you analyze small DNA molecules, and for large molecules, a solution as low as 0.7% can be used. |
|||
#Carefully bring the solution just to the boil to dissolve the agarose, preferably in a [[microwave oven]]. |
|||
#Let the solution cool down to about 60 °C at room temperature, or water bath. Stir or swirl the solution while cooling. |
|||
''Wear gloves from here on, [[ethidium bromide]] is a [[mutagen]], for more information on safety see [[ethidium bromide]]'' |
|||
#Add 5 µl ethidium bromide stock (10 mg/ml) per 100 ml gel solution for a final concentration of 0.5 ug/ml. Be very careful when handling the concentrated stock. Some researchers prefer not to add ethidium bromide to the gel itself, instead soaking the gel in an ethidium bromide solution after running. |
|||
#Stir the solution to disperse the ethidium bromide, then pour it into the gel rack. |
|||
#Insert the comb at one side of the gel, about 5-10 mm from the end of the gel. |
|||
#When the gel has cooled down and become solid, carefully remove the comb. The holes that remain in the gel are the wells or slots. |
|||
#Put the gel, together with the rack, into a tank with TAE. [[Ethidium bromide]] at the same concentration can be added to the buffer. The gel must be completely covered with TAE, with the slots at the end electrode that will have the negative current. |
|||
===Procedure=== |
|||
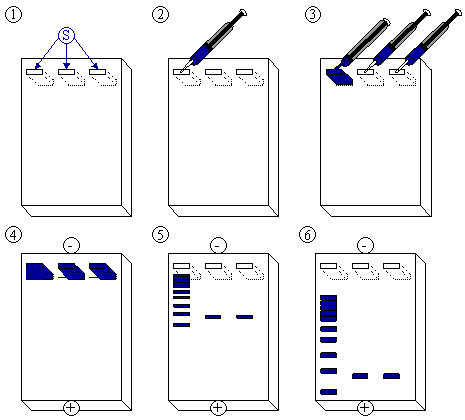
After the gel has been prepared, use a micropipette to inject about 2.5 µl of stained DNA (a DNA ladder is also highly recommended). Close the lid of the electrophoresis chamber and apply current (typically 100 V for 30 minutes with 15 ml of gel). The colored dye in the DNA ladder and DNA samples acts as a "front wave" that runs faster than the DNA itself. When the "front wave" approaches the end of the gel, the current is stopped. The DNA is stained with ethidium bromide, and is then visible under [[ultraviolet]] light. |
|||
#The agarose gel with three slots/wells (S). |
|||
#Injection of DNA ladder ([[molecular weight]] markers) into the first slot. |
|||
#DNA ladder injected. Injection of samples into the second and third slot. |
|||
#A current is applied. The DNA moves toward the positive [[anode]] due to the negative charges on its [[phosphate]] backbone. |
|||
#Small DNA strands move fast, large DNA strands move slowly through the gel. The DNA is not normally visible during this process, so the marker dye is added to the DNA to avoid the DNA being run entirely off the gel. The marker dye has a low molecular weight, and migrates faster than the DNA, so as long as the marker has not run past the end of the gel, the DNA will still be in the gel. |
|||
#Add the color marker dye to the DNA ladder. |
|||
[[Image:Gel electrophoresis 1.jpg|thumb|Agarose gel with samples loaded in the slots, before the electrophoresis process]] |
|||
[[Image:Gel electrophoresis 2.jpg|thumb|A pattern of DNA-bands under UV light]] |
|||
[[Image:Agarose-Gelelektrophorese.png|frame|none|Figure 1: Schematic drawing of the electrophoresis process, see text for description of steps]] |
|||
===References=== |
|||
{{reflist}} |
|||
==See also== |
|||
*[[gel electrophoresis]] |
|||
*[[SDS-polyacrylamide gel electrophoresis]] |
|||
*[[Southern blot]] |
|||
*[[Northern blot]] |
|||
*[[PCR]] |
|||
*[[Restriction endonuclease]] |
|||
==External links== |
|||
*[http://arbl.cvmbs.colostate.edu/hbooks/genetics/biotech/gels/index.html How to run a DNA or RNA gel] |
|||
*[http://arbl.cvmbs.colostate.edu/hbooks/genetics/biotech/gels/virgel.html Animation of gel analysis of DNA restriction fragments] |
|||
*[http://www.umd.umich.edu/labtv/modules/agarosegel/agarose.html Detailed description and movies of the preparation and uses of agarose gels] |
|||
*[http://web.mit.edu/7.02/virtual_lab/RDM/RDM1virtuallab.html Step by step photos of running a gel and extracting DNA] |
|||
[[Category:Biological techniques and tools]] |
|||
[[Category:Molecular biology]] |
|||
[[Category:Electrophoresis]] |
|||
[[Category:Polymerase chain reaction]] |
|||
[[de:Agarose-Gelelektrophorese]] |
|||
[[fr:Électrophorèse en gel d'agarose]] |
|||
[[it:Elettroforesi su gel di agarosio]] |
|||
[[ja:アガロースゲル電気泳動]] |
|||
Revision as of 20:20, 4 December 2008
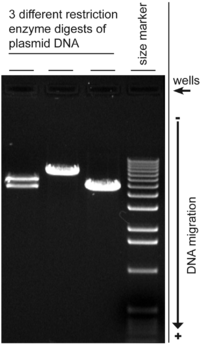
Agarose gel electrophoresis is a method used in biochemistry and molecular biology to separate DNA, or RNA molecules by size. This is achieved by moving negatively charged nucleic acid molecules through an agarose matrix with an electric field (electrophoresis). Shorter molecules move faster and migrate farther than longer ones.[1]
Applications
- Estimation of the size of DNA molecules following restriction enzyme digestion, e.g. in restriction mapping of cloned DNA.
- Analysis of PCR products, e.g. in molecular genetic diagnosis or genetic fingerprinting
- Separation of restricted genomic DNA prior to Southern transfer, or of RNA prior to Northern transfer.
The advantages are that the gel is easily poured, does not denature the samples. The samples can also be recovered.
The disadvantages are that gels can melt during electrophoresis, the buffer can become exhausted, and different forms of genetic material may run in unpredictable forms.
Factors affecting migration
The most important factor is the length of the DNA molecule, smaller molecules travel farther. But conformation of the DNA molecule is also a factor. To avoid this problem linear molecules are usually separated, usually DNA fragments from a restriction digest, linear DNA PCR products, or RNAs.
Increasing the agarose concentration of a gel reduces the migration speed and enables separation of smaller DNA molecules. The higher the voltage, the faster the DNA moves. But voltage is limited by the fact that it heats and ultimately causes the gel to melt. High voltages also decrease the resolution (above about 5 to 8 V/cm).
Conformations of a DNA plasmid that has not been cut with a restriction enzyme will move with different speeds (slowest to fastest): nicked or open circular, linearised, or supercoiled plasmid.
Visualisation: Ethidium Bromide (EtBr) and dyes
The most common dye used to make DNA or RNA bands visible for agarose gel electrophoresis is ethidium bromide, usually abbreviated as EtBr. It fluoresces under UV light when intercalated into DNA (or RNA). By running DNA through an EtBr-treated gel and visualizing it with UV light, any band containing more than ~20ng DNA becomes distinctly visible. EtBr is a known carcinogen, however, and safer alternatives are available.
SYBR Green I is another dsDNA stain, produced by Invitrogen. It is more expensive, but 25 times more sensitive, and possibly safer than EtBr, though there is no data addressing its mutagenicity or toxicity in humans.[2]
SYBR Safe is a variant of SYBR Green that has been shown to have low enough levels of mutagenicity and toxicity to be deemed nonhazardous waste under U.S. Federal regulations.[3] It has similar sensitivity levels to EtBr,[3] but, like SYBR Green, is significantly more expensive.
Since EtBr stained DNA is not visible in natural light, scientists mix DNA with negatively charged loading buffers before adding the mixture to the gel. Loading buffers are useful because they are visible in natural light (as opposed to UV light for EtBr stained DNA), and they co-sediment with DNA (meaning they move at the same speed as DNA of a certain length). Xylene cyanol and Bromophenol blue are common loading buffers; they run about the same speed as DNA fragments that are 5000 bp and 300 bp in length respectively, but the precise position varies with percentage of the gel. Other less frequently used progress markers are Cresol Red and Orange G which run at about 125 bp and 50 bp.
Percent agarose and resolution limits
Agarose gel electrophoresis can be used for the separation of DNA fragments ranging from 50 base pair to several megabases (millions of bases) using specialized apparatus. The distance between DNA bands of a given length is determined by the percent agarose in the gel. In general lower concentrations of agarose are better for larger molecules because they result in greater separation between bands that are close in size. The disadvantage of higher concentrations is the long run times (sometimes days). Instead high percentage agarose gels should be run with a pulsed field electrophoresis (PFE), or field inversion electrophoresis.
Most agarose gels are made with between 0.7% (good separation or resolution of large 5–10kb DNA fragments) and 2% (good resolution for small 0.2–1kb fragments) agarose dissolved in electrophoresis buffer. Some people go as high as 3% for separating very tiny fragments but a vertical polyacrylamide gel is more appropriate in this case. Low percentage gels are very weak and may break when you try to lift them. High percentage gels are often brittle and do not set evenly. 1% gels are common for many applications.
Buffers
There are a number of buffers used for agarose electrophoresis. The most common being: tris acetate EDTA (TAE), Tris/Borate/EDTA (TBE) and Sodium borate (SB). TAE has the lowest buffering capacity but provides the best resolution for larger DNA. This means a lower voltage and more time, but a better product. SB is relatively new and is ineffective in resolving fragments larger than 5 kbp; However, with its low conductivity, a much higher voltage could be used (up to 35 V/cm), which means a shorter analysis time for routine electrophoresis. As low as one base pair size difference could be resolved in 3% agarose gel with an extremely low conductivity medium (1 mM Lithium borate).[4]
Analysis
After electrophoresis the gel is illuminated with an ultraviolet lamp (usually by placing it on a light box, while using protective gear to limit exposure to ultraviolet radiation) to view the DNA bands. The ethidium bromide fluoresces reddish-orange in the presence of DNA. The DNA band can also be cut out of the gel, and can then be dissolved to retrieve the purified DNA. The gel can then be photographed usually with a digital or polaroid camera. Although the stained nucleic acid fluoresces reddish-orange, images are usually shown in black and white (see figures).
Gel electrophoresis research often takes advantage of software-based image analysis tools, such as ImageJ.
| 1 | 2 | 3 |
|---|---|---|
 |
 |
 |
Typical method
Materials
Typically 10-30 μl/sample of the DNA fragments to separate are obtained, as well as a mixture of DNA fragments (usually 10-20) of known size (after processing with DNA size markers either from a commercial source or prepared manually).
- Buffer solution, usually TBE buffer or TAE 1.0x, pH 8.0
- Agarose
An ultraviolet-fluorescent dye, ethidium bromide, (5.25 mg/ml in H2O). The stock solution be careful handling this.
- Alternative dyes may be used, such as SYBR Green.
- Nitrile rubber gloves
- Latex gloves do not protect well from ethidium bromide
- A color marker dye containing a low molecular weight dye such as "bromophenol blue" (to enable tracking the progress of the electrophoresis) and glycerol (to make the DNA solution denser so it will sink into the wells of the gel).
- A gel rack
- A "comb"
- Power Supply
- UV lamp or UV lightbox or other method to visualize DNA in the gel
Preparation
There are several methods for preparing gels. A common example is shown here. Other methods might differ in the buffering system used, the sample size to be loaded, the total volume of the gel (typically thickness is kept to a constant amount while length and breadth are varied as needed). Most agarose gels used in modern biochemistry and molecular biology are prepared and run horizontally.
- Make a 1% agarose solution in 100ml TAE, for typical DNA fragments (see figures). A solution of up to 2-4% can be used if you analyze small DNA molecules, and for large molecules, a solution as low as 0.7% can be used.
- Carefully bring the solution just to the boil to dissolve the agarose, preferably in a microwave oven.
- Let the solution cool down to about 60 °C at room temperature, or water bath. Stir or swirl the solution while cooling.
Wear gloves from here on, ethidium bromide is a mutagen, for more information on safety see ethidium bromide
- Add 5 µl ethidium bromide stock (10 mg/ml) per 100 ml gel solution for a final concentration of 0.5 ug/ml. Be very careful when handling the concentrated stock. Some researchers prefer not to add ethidium bromide to the gel itself, instead soaking the gel in an ethidium bromide solution after running.
- Stir the solution to disperse the ethidium bromide, then pour it into the gel rack.
- Insert the comb at one side of the gel, about 5-10 mm from the end of the gel.
- When the gel has cooled down and become solid, carefully remove the comb. The holes that remain in the gel are the wells or slots.
- Put the gel, together with the rack, into a tank with TAE. Ethidium bromide at the same concentration can be added to the buffer. The gel must be completely covered with TAE, with the slots at the end electrode that will have the negative current.
Procedure
After the gel has been prepared, use a micropipette to inject about 2.5 µl of stained DNA (a DNA ladder is also highly recommended). Close the lid of the electrophoresis chamber and apply current (typically 100 V for 30 minutes with 15 ml of gel). The colored dye in the DNA ladder and DNA samples acts as a "front wave" that runs faster than the DNA itself. When the "front wave" approaches the end of the gel, the current is stopped. The DNA is stained with ethidium bromide, and is then visible under ultraviolet light.
- The agarose gel with three slots/wells (S).
- Injection of DNA ladder (molecular weight markers) into the first slot.
- DNA ladder injected. Injection of samples into the second and third slot.
- A current is applied. The DNA moves toward the positive anode due to the negative charges on its phosphate backbone.
- Small DNA strands move fast, large DNA strands move slowly through the gel. The DNA is not normally visible during this process, so the marker dye is added to the DNA to avoid the DNA being run entirely off the gel. The marker dye has a low molecular weight, and migrates faster than the DNA, so as long as the marker has not run past the end of the gel, the DNA will still be in the gel.
- Add the color marker dye to the DNA ladder.


References
- ^ Sambrook J, Russel DW (2001). Molecular Cloning: A Laboratory Manual 3rd Ed. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY.
- ^ SYBR Green I Nucleic Acid Gel Stain
- ^ a b SYBR Safe DNA Gel Stain
- ^ Brody JR, Calhoun ES, Gallmeier E, Creavalle TD, Kern SE (2004). Ultra-fast high-resolution agarose electrophoresis of DNA and RNA using low-molarity conductive media. Biotechniques. 37:598-602. [1]
See also
- gel electrophoresis
- SDS-polyacrylamide gel electrophoresis
- Southern blot
- Northern blot
- PCR
- Restriction endonuclease
