Azodicarbonamide: Difference between revisions
m Dating maintenance tags: {{Citation needed}} |
WHO; Subway petition |
||
| Line 44: | Line 44: | ||
}} |
}} |
||
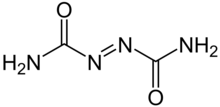
'''Azodicarbonamide''', or '''azobisformamide''', is a chemical compound with the molecular formula C<sub>2</sub>H<sub>4</sub>O<sub>2</sub>N<sub>4</sub>.<ref>{{Cite web|url=http://www.inchem.org/documents/cicads/cicads/cicad16.htm#PartNumber:2|title=Azodicarbonamide (CICADS)|publisher=[[International Programme on Chemical Safety]]|work=Inchem|accessdate=2010-08-14| archiveurl= http://web.archive.org/web/20100824055553/http://www.inchem.org/documents/cicads/cicads/cicad16.htm| archivedate= 24 August 2010 <!--DASHBot-->| deadurl= no}}</ref> It is a yellow to orange red, odorless, crystalline powder. As a [[food additive]], it is known by the [[E number]] '''E927'''. |
'''Azodicarbonamide''', or '''azobisformamide''', is a chemical compound with the molecular formula C<sub>2</sub>H<sub>4</sub>O<sub>2</sub>N<sub>4</sub>.<ref>{{Cite web|url=http://www.inchem.org/documents/cicads/cicads/cicad16.htm#PartNumber:2|title=Azodicarbonamide (CICADS)|publisher=[[International Programme on Chemical Safety]]|work=Inchem|accessdate=2010-08-14| archiveurl= http://web.archive.org/web/20100824055553/http://www.inchem.org/documents/cicads/cicads/cicad16.htm| archivedate= 24 August 2010 <!--DASHBot-->| deadurl= no}} Also published by [[World Health Organization]], Geneva, 1999.</ref> It is a yellow to orange red, odorless, crystalline powder. As a [[food additive]], it is known by the [[E number]] '''E927'''. |
||
==Use as a food additive== |
==Use as a food additive== |
||
| Line 71: | Line 71: | ||
==External links== |
==External links== |
||
* [http://www.cdc.gov/niosh/ipcsneng/neng0380.html International Chemical Safety Card] |
* [http://www.cdc.gov/niosh/ipcsneng/neng0380.html International Chemical Safety Card] |
||
* [http://foodbabe.com/subway/ Petition] to have [[Subway (restaurant)|Subway]] restaurants drop azodicarbonamide from breads, 2014. |
|||
{{E number infobox 920-929}} |
{{E number infobox 920-929}} |
||
Revision as of 04:20, 5 February 2014
| |
| Names | |
|---|---|
| IUPAC name
Carbamoyliminourea
| |
| Other names
Azodicarboxamide; Azobisformamide; C,C'-Azodi(formamide); Diazenedicarboxamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.229 |
| E number | E927a (glazing agents, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H4N4O2 | |
| Molar mass | 116.080 g·mol−1 |
| Appearance | Yellow to orange/red crystalline powder |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Azodicarbonamide, or azobisformamide, is a chemical compound with the molecular formula C2H4O2N4.[1] It is a yellow to orange red, odorless, crystalline powder. As a food additive, it is known by the E number E927.
Use as a food additive
Azodicarbonamide is used as a food additive, a flour bleaching agent and improving agent. It reacts with moist flour as an oxidizing agent.[2] The main reaction product is biurea,[3] a derivative of urea, which is stable during baking. Secondary reaction products include semicarbazide[4] and ethyl carbamate.[5] The United States permits the use of azodicarbonamide at levels up to 45 ppm.[6] In Australia[citation needed] the use of azodicarbonamide as a food additive is banned. In Singapore, use is punishable by up to 15 years in prison and a fine of $450,000[citation needed].
Other uses
The principal use of azodicarbonamide is in the production of foamed plastics as an additive. The thermal decomposition of azodicarbonamide results in the evolution of nitrogen, carbon monoxide, carbon dioxide, and ammonia gases, which are trapped in the polymer as bubbles to form a foamed article.
Azodicarbonamide as used in plastics, synthetic leather and other uses can be pure or modified. This is important because modification affects the reaction temperatures. Pure azodicarbonamide generally reacts around 200 °C, but there are some products that the reaction temperature must be lower, depending on the application. In the plastic, leather and other industries, modified azodicarbonamide (average decomposition temperature 170 °C) contains additives that accelerate the reaction or react at lower temperatures.
Azodicarbonamide as a blowing agent in plastics has been banned in Europe since August 2005 for the manufacture of plastic articles that are intended to come into direct contact with food.[7]
Safety
In the United States, azodicarbonamide has generally recognized as safe (GRAS) status and is allowed to be added to flour at levels up to 45 ppm.[8]
In the UK, the Health and Safety Executive has identified azodicarbonamide as a respiratory sensitizer (a possible cause of asthma) and determined that products should be labeled with "May cause sensitisation by inhalation."[9]
Toxicological studies of the reactions of azodicarbonamide show that it is rapidly converted in dough to biurea, which is a stable compound not decomposed upon cooking.[10]
See also
References
- ^ "Azodicarbonamide (CICADS)". Inchem. International Programme on Chemical Safety. Archived from the original on 24 August 2010. Retrieved 2010-08-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) Also published by World Health Organization, Geneva, 1999. - ^ "Azodicarbonamide FCC Grade (98%)". Garuda International, Inc. 2009-02-13. Retrieved 2010-08-14.
- ^ Joiner, Robert; Vidal, Frederick; Marks, Henry (September 1963). "A New Powdered Agent for Flour Maturing". Cereal Chemistry. 40: 539–553.
- ^ Becalski A, Lau BP, Lewis D, Seaman SW (2004-09-10). "Semicarbazide formation in azodicarbonamide-treated flour: a model study". Journal of Agricultural and Food Chemistry. 52 (18). Journal of Agricultural and Food Chemistry: 5730–4. doi:10.1021/jf0495385. PMID 15373416.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cañas, BJ; Diachenko, GW; Nyman, PJ (January 1997). "Ethyl carbamate levels resulting from azodicarbonamide use in bread". Food Additives & Contaminants. 14 (1): 89–94. doi:10.1080/02652039709374501. PMID 9059587.
- ^ "21CFR172.806" (Document). April 1, 2012.
{{cite document}}: Cite document requires|publisher=(help); Unknown parameter|url=ignored (help); Unknown parameter|work=ignored (help) - ^ "COMMISSION DIRECTIVE 2004/1/EC of 6 January 2004 amending Directive 2002/72/EC as regards the suspension of the use of azodicarbonamide as blowing agent". Official Journal of the European Union. 2004-01-13. Retrieved 2011-03-10.
- ^ "CFR - Code of Federal Regulations Title 21". United States Department of Health and Human Services. 2009-04-01. Retrieved 2010-08-14.
- ^ "Substances causing/worsening asthma". UK Occupational Health and Safety. WorkSafe Victoria. Retrieved 2010-08-14.
- ^ "054. Azodicarbonamide (FAO Nutrition Meetings Report Series 40abc)". FAO/WHO. 1966-10-18. Archived from the original on 23 August 2010. Retrieved 2010-08-14.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
External links
- International Chemical Safety Card
- Petition to have Subway restaurants drop azodicarbonamide from breads, 2014.

