Wave function: Difference between revisions
→Position-space wave function: links and slight trim |
move "normalization example" down to the examples section |
||
| Line 87: | Line 87: | ||
where {{math|''x''}} is position and {{math|''t''}} is time. This is a [[complex-valued function|complex-valued]] function of two real variables {{math|''x''}} and {{math|''t''}}. |
where {{math|''x''}} is position and {{math|''t''}} is time. This is a [[complex-valued function|complex-valued]] function of two real variables {{math|''x''}} and {{math|''t''}}. |
||
If interpreted as a [[probability amplitude]], the square modulus of the wave function is the positive real number |
If interpreted as a [[probability amplitude]], the square [[absolute value|modulus]] of the wave function is the positive real number |
||
:<math> \left|\Psi(x, t)\right|^2 = {\Psi(x, t)}^{*}\Psi(x, t) = \rho(x, t) </math> |
:<math> \left|\Psi(x, t)\right|^2 = {\Psi(x, t)}^{*}\Psi(x, t) = \rho(x, t) </math> |
||
| Line 143: | Line 143: | ||
:<math>\langle \Phi_1 , \Phi_2 \rangle = \int\limits_{-\infty}^\infty d p \, \Phi_1^*(p, t)\Phi_2(p, t) \,,</math> |
:<math>\langle \Phi_1 , \Phi_2 \rangle = \int\limits_{-\infty}^\infty d p \, \Phi_1^*(p, t)\Phi_2(p, t) \,,</math> |
||
All the previous remarks on superposition, inner products, normalization, etc. apply similarly. In particular, if the particle's momentum is [[measurement in quantum mechanics|measured]], the result is not deterministic, but is described by a probability distribution: |
|||
:<math>P_{a\le p\le b} (t) = \int\limits_a^b d p \, |\Phi(p,t)|^2 \,,</math> |
:<math>P_{a\le p\le b} (t) = \int\limits_a^b d p \, |\Phi(p,t)|^2 \,,</math> |
||
and the normalization condition is |
and the normalization condition is: |
||
:<math> \langle \Phi , \Phi \rangle = \int\limits_{-\infty}^{\infty} d p \, \left | \Phi \left ( p, t \right ) \right |^2 = 1\,.</math> |
:<math> \langle \Phi , \Phi \rangle = \int\limits_{-\infty}^{\infty} d p \, \left | \Phi \left ( p, t \right ) \right |^2 = 1\,.</math> |
||
| Line 165: | Line 165: | ||
and the equivalent space is referred to as [[Momentum space|{{math|''k''}}-space]]. Again it makes no difference which is used since {{math|''p''}} and {{math|''k''}} are equivalent – up to a constant. In practice, the position-space wave function is used much more often than the momentum-space wave function. |
and the equivalent space is referred to as [[Momentum space|{{math|''k''}}-space]]. Again it makes no difference which is used since {{math|''p''}} and {{math|''k''}} are equivalent – up to a constant. In practice, the position-space wave function is used much more often than the momentum-space wave function. |
||
=== Example of normalization === |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
== Definitions (other cases) == |
== Definitions (other cases) == |
||
| Line 362: | Line 341: | ||
:<math> \sum_{i=1}^n | \varepsilon_i \rangle \langle \varepsilon_i | = 1 \,,\quad \int d\varepsilon \, | \varepsilon \rangle \langle \varepsilon | = 1 \, </math> |
:<math> \sum_{i=1}^n | \varepsilon_i \rangle \langle \varepsilon_i | = 1 \,,\quad \int d\varepsilon \, | \varepsilon \rangle \langle \varepsilon | = 1 \, </math> |
||
for the discrete and continuous orthonormal bases, respectively. An orthonormal set of kets form bases if and only if they satisfy these relations.<ref name="Schaum QM"/> In each case, the equality to unity means this is an [[identity operator]]; its action on any state leaves it unchanged. Multiplying any state on the right of these gives the representation of the state {{math|{{ket|Ψ}}}} in the basis. The inner product of a first state {{math|{{ket| |
for the discrete and continuous orthonormal bases, respectively. An orthonormal set of kets form bases if and only if they satisfy these relations.<ref name="Schaum QM"/> In each case, the equality to unity means this is an [[identity operator]]; its action on any state leaves it unchanged. Multiplying any state on the right of these gives the representation of the state {{math|{{ket|Ψ}}}} in the basis. The inner product of a first state {{math|{{ket|Ψ<sub>1</sub>}}}}}} with a second {{math|{{ket|Ψ<sub>2</sub>}}}} can also be obtained by multiplying {{math|{{math|{{ket|Ψ<sub>1</sub>}}}}}} on the left and {{math|{{ket|Ψ<sub>2</sub>}}}} on the right of the relevant completeness condition. |
||
=== Inner product === |
=== Inner product === |
||
| Line 414: | Line 393: | ||
=== Wave function collapse === |
=== Wave function collapse === |
||
The physical meaning of the components of {{math|{{ket|Ψ}}}} is given by the ''wave function [[collapse postulate]] also known as [[Wave function collapse]] |
The physical meaning of the components of {{math|{{ket|Ψ}}}} is given by the ''wave function [[collapse postulate]]'', also known as [[Wave function collapse]]. If the observable(s) {{math|''ε''}} (momentum and/or spin, position and/or spin, etc.) corresponding to states {{math|{{ket|''ε<sub>i</sub>''}}}} has distinct and definite values, {{math|''λ<sub>i</sub>''}}, and a measurement of that variable is performed on a system in the state {{math|{{ket|Ψ}}}} then the probability of measuring {{math|''λ<sub>i</sub>''}} is {{math|{{abs|{{bra-ket|''ε<sub>i</sub>''|Ψ}}}}<sup>2</sup>}}. If the measurement yields {{math|''λ<sub>i</sub>''}}, the system "collapses" to the state {{math|{{ket|''ε<sub>i</sub>''}}}}, irreversibly and instantaneously. |
||
=== Time dependence === |
=== Time dependence === |
||
| Line 511: | Line 490: | ||
==Examples== |
==Examples== |
||
| ⚫ | |||
{{main|Free particle}} |
|||
A free particle in 3d with [[wave vector]] {{math|'''k'''}} has a wave function |
|||
:<math>\Psi (\mathbf{r},t) = A e^{i(\mathbf{k}\cdot\mathbf{r}-\omega t)}</math> |
|||
| ⚫ | |||
{{main|Particle in a box}} |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
===One-dimensional quantum tunnelling=== |
===One-dimensional quantum tunnelling=== |
||
| Line 530: | Line 540: | ||
===Other=== |
===Other=== |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
* [[Finite square well]] |
* [[Finite square well]] |
||
* [[Delta potential]] |
* [[Delta potential]] |
||
Revision as of 19:22, 14 March 2014

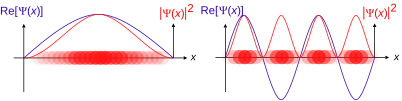
A wave function or wavefunction (also named a state function) in quantum mechanics describes the quantum state of a particle and how it behaves, thus a central quantity in quantum mechanics. Typically, its values are complex numbers and, for a single particle, it is a function of space and time. The Schrödinger equation describes how the wave function evolves over time. The wave function behaves qualitatively like other waves, like water waves or waves on a string, because the Schrödinger equation is mathematically a type of wave equation. This explains the name "wave function", and gives rise to wave–particle duality.
The most common symbols for a wave function are ψ or Ψ (lower-case and capital psi).
Although values of ψ are complex numbers, |ψ|2 is real corresponding by Max Born's proposal to the probability density of finding a particle in a given place at a given time, if the particle's position is to be measured. Louis de Broglie in his later years proposed a real-valued wave function connected to the complex wave function by a proportionality constant and developed the de Broglie–Bohm theory.
The unit of measurement for ψ depends on the system. For one particle in three dimensions, its units are [length]−3/2. These unusual units are required so that an integral of |ψ|2 over a region of three-dimensional space is a unitless probability (the probability that the particle is in that region). For different numbers of particles and/or dimensions, the units may be different and can be found by dimensional analysis.[1]
Although the wave function contains information, it is a complex-valued quantity; only its relative phase and relative magnitude can be measured. It does not directly tell anything about the magnitudes or directions of measurable observables, one has to apply quantum operators to the wave function ψ and find the eigenvalues which correspond to sets of possible results of measurement.
Historical background
| Part of a series of articles about |
| Quantum mechanics |
|---|
In the 1920s and 1930s, quantum mechanics was developed using calculus and linear algebra. Those who used the techniques of calculus included Louis de Broglie, Erwin Schrödinger, and others, developing "wave mechanics". Those who applied the methods of linear algebra included Werner Heisenberg, Max Born, and others, developing "matrix mechanics". Schrödinger subsequently showed that the two approaches were equivalent.[2] In each case, the wave function was at the centre of attention in two forms, giving quantum mechanics its unity.
In 1905 Planck postulated the proportionality between the frequency of a photon and its energy, in the Planck–Einstein equation, E = hf. In 1925, De Broglie published the symmetric relation between momentum and wavelength, λ = h/p, now called the De Broglie relation. These equations represent wave–particle duality. In 1926, Schrödinger published the famous wave equation now named after him, indeed the Schrödinger equation, based on classical energy conservation using quantum operators and the de Broglie relations such that the solutions of the equation are the wave functions for the quantum system. Later Pauli invented the Pauli equation that adds a description of electron's spin and magnetic dipole. However, no one, even Schrödinger and De Broglie, were clear on how to interpret it.[3] Around 1924–27, Max Born, Heisenberg, Bohr and others provided the perspective of probability amplitude.[4] This is the Copenhagen interpretation of quantum mechanics. There are many other interpretations of quantum mechanics, but this is considered the most important – since quantum calculations can be understood.
In 1927, Hartree and Fock made the first step in an attempt to solve the N-body wave function, and developed the self-consistency cycle: an iterative algorithm to approximate the solution. Now it is also known as the Hartree–Fock method.[5] The Slater determinant and permanent (of a matrix) was part of the method, provided by John C. Slater.
Interestingly, Schrödinger did encounter an equation for which the wave function satisfied relativistic energy conservation before he published the non-relativistic one, but it led to unacceptable consequences; negative probabilities and negative energies, so he discarded it. In 1927, Klein, Gordon and Fock also found it, but taking a step further: incorporated the electromagnetic interaction into it and proved it was Lorentz-invariant. De Broglie also arrived at exactly the same equation in 1928. This relativistic wave equation is now known most commonly as the Klein–Gordon equation.[6]
In 1927, Pauli phenomenologically found a non-relativistic equation to describe spin-1/2 particles in electromagnetic fields, now called the Pauli equation. Pauli found the wave function was not a single complex number, but two complex numbers, which correspond to the spin +1/2 and −1/2 states of the fermion. Soon after in 1928, Dirac found an equation from the first successful unification of special relativity and quantum mechanics applied to the electron – now called the Dirac equation. He found the wave function for this equation could not be a single complex number, but a four-component spinor.[5] Spin automatically entered into the properties of the wave function. Later other wave equations were developed: see relativistic wave equations for further information.
Wave functions and function spaces
Functional analysis is commonly used to formulate the wave function with a necessary mathematical precision; usually they are quadratically integrable functions (at least locally) because it is compatible with the Hilbert space formalism mentioned below. The set on which their function space is defined is the configuration space of the system. In many situations it is an Euclidean space, that implies that wave functions are functions of several real variables. Superficially, this formalism is simple to understand for the following reasons.
- If the wave function is to change throughout space and time, one would expect the wave function to be a function of the position and time coordinates. It is solved from the Schrödinger equation (or other relativistic wave equations), a linear partial differential equation:
- Functions can easily describe wave-like motion, using periodic functions, and Fourier analysis can be readily done.
- Functions are easy to produce, visualize, and interpret, because of the pictorial nature of the graph of a function. One can plot curves, surfaces, contour lines, more generally any level sets. If the situation is in a high number of dimensions – one can analyze the function in a lower dimensional slice to see the behavior of the function within that confined region.
For concreteness and simplicity, in this article, when coordinates are needed we use Cartesian coordinates so that r is short for (x, y, z), although spherical polar coordinates and other orthogonal coordinates are often useful to solve the Schrödinger equation for potentials with certain geometric symmetries, in which case the position and wave function is expressed in these coordinates.
One does not have to define wave functions necessarily on real spaces: appropriate function spaces can be defined wherever a measure can provide integration. Operator theory and linear algebra, as shown next, can deal with situations where the real analysis is not applicable.
Requirements

The following constraints on the wave function are formulated for the calculations and physical interpretation to make sense:[7][8]
- It must everywhere be a continuous function, and continuously differentiable.
- It must everywhere satisfy the relevant normalization condition, because the particle or system of particles exists somewhere with 100% certainty. For this to be so, the wave function must be square integrable.
A requirement less restrictive is that the wave function must belong to the Sobolev space W1,2. It means that it is differentiable in the sense of distributions, and its gradient is square-integrable. This relaxation is necessary for potentials that are not functions but are distributions, such as the dirac delta function.
If these requirements are not met, it is not possible to interpret the wave function as a probability amplitude.[9]
Definition (one spinless particle in 1d)
For now, consider the simple case of a single particle, without spin, in one spatial dimension. (More general cases are discussed below).
Position-space wave function
The state of such a particle is completely described by its wave function:
where x is position and t is time. This is a complex-valued function of two real variables x and t.
If interpreted as a probability amplitude, the square modulus of the wave function is the positive real number
interpreted as the probability density that the particle is at x, rather than some other location. The star * indicates complex conjugate. If the particle's position is measured, its location is not deterministic, but is described by a probability distribution. The probability that its position x will be in the interval a ≤ x ≤ b is the integral of the density over this interval:
where t is the time at which the particle was measured. This leads to the normalization condition:
because if the particle is measured, there is 100% probability that it will be somewhere.
Since the Schrödinger equation is linear, if any number of wave functions Ψn for n = 1, 2, ... are solutions of the equation, then so is their sum, and their scalar multiples by complex numbers an (taking scalar multiplication and addition together is known as a linear combination):
This is the superposition principle. Multiplying a wave function Ψ by any nonzero constant complex number c to obtain cΨ does not change any information about the quantum system, because c cancels in the Schrödinger equation for cΨ. All that happens is that any normalization constants will be rescaled.
Since linear combinations of wave functions obtain more wave functions, the set of all wave functions forms a complex vector space over the field of complex numbers (more details are given later).
The inner product of two wave functions Ψ1(x, t) and Ψ2(x, t) is useful and important for a number of reasons, and can be defined as the complex number (at time t):
In the Copenhagen interpretation, the modulus squared of this complex number gives a real number
which is interpreted as the probability of the wave function Ψ2 "collapsing" to the new wave function Ψ1.
Although the inner product of two wave functions is a complex number, the inner product of a wave function Ψ with itself,
is always a positive real number. The number ||Ψ|| is called the norm of the wave function Ψ, and is not the same as the modulus |Ψ|. A wave function is normalized if:
This is the normalization condition above, but written in a form that generalizes easily to more complicated systems (see below). If Ψ is not normalized, then dividing by its norm gives the normalized function Ψ/||Ψ||.
A set of wave functions Ψ1, Ψ2, ... are orthonormal if they are each normalized (inner product each wave function with itself is 1) and are all orthogonal to each other (inner product of any two different wave functions is zero):
where m and n each take values 1, 2, ..., and δmn is the Kronecker delta (+1 for m = n and 0 for m ≠ n).
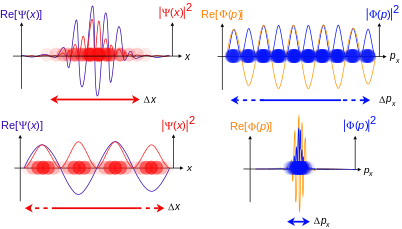
Momentum-space wave function
The particle also has a wave function in momentum space:
where p is the momentum in one dimension, which can be any value from −∞ to +∞, and t is time. Analogous to the position case, the inner product of two wave functions Φ1(p, t) and Φ2(p, t) can be defined as:
All the previous remarks on superposition, inner products, normalization, etc. apply similarly. In particular, if the particle's momentum is measured, the result is not deterministic, but is described by a probability distribution:
and the normalization condition is:
Relation between wave functions
The position-space and momentum-space wave functions are Fourier transforms of each other, therefore both contain the same information, and either one alone is sufficient to calculate any property of the particle. For one dimension:[10]
Sometimes the wave-vector k is used in place of momentum p, since they are related by the de Broglie relation
and the equivalent space is referred to as k-space. Again it makes no difference which is used since p and k are equivalent – up to a constant. In practice, the position-space wave function is used much more often than the momentum-space wave function.
Definitions (other cases)

General forms
Following are the general forms of the wave function for systems in higher dimensions and more particles, as well as including other degrees of freedom than position coordinates or momentum components.
The position-space wave function of a single particle in three spatial dimensions is similar to the case of one spatial dimension above:
where r is the position vector in three-dimensional space, and t is time. As always Ψ(r, t) is a complex number, for this case a complex-valued function of four real variables.
If there are many particles, in general there is only one wave function, not a separate wave function for each particle. The fact that one wave function describes many particles is what makes quantum entanglement and the EPR paradox possible. The position-space wave function for N particles is written:[5]
where ri is the position of the ith particle in three-dimensional space, and t is time. Altogether, this is a complex-valued function of 3N + 1 real variables.
For a particle with spin, the wave function can be written in "position–spin space" as:
where r is a position in three-dimensional space, t is time, and sz is the spin projection quantum number along the z axis. (The z axis is an arbitrary choice; other axes can be used instead if the wave function is transformed appropriately, see below.) The sz parameter, unlike r and t, is a discrete variable. For example, for a spin-1/2 particle, sz can only be +1/2 or −1/2, and not any other value. (In general, for spin s, sz can be s, s − 1, ... , −s.) It is convenient to write the wave function as a column vector, in which there are as many entries in the column vector as there are allowed values of sz, and the entries are indexed by the spin quantum number:[11]
For spinless wave functions, Ψ*Ψ is the complex scalar needed for normalization, but for spin wave functions the Hermitian conjugate (complex conjugate transpose of the column vector into a row vector) is required to obtain the real number Ψ†Ψ (the ordering of Ψ† and Ψ does matter - see matrix multiplication).
Since the position and spin degrees of freedom of the particle are separate from one another, the wave function is a product of a purely position space wave function ψ and a purely spin-dependent function ξ:[12]
The wave function for N particles each with spin is:
and the wave function is a product of a position space wave function ψ and a spin-dependent function ξ:[13]
Inner products and probability interpretations for many particles in higher dimensions
The formulae for the inner products are integrals over all coordinates or momenta and sums over all spin quantum numbers. That is, for one spinless particle in 3d the inner product of two wave functions can be defined as the complex number:
while for many spinless particles in 3d:
(altogether, this is N three-dimensional volume integrals over the regions Ri for i = 1, ..., N with differential volume elements d3ri, also written "dVi" or "dxi dyi dzi"). For one particle with spin in 3d:
and for the general case of N particles with spin in 3d:
(altogether, N three-dimensional volume integrals over the regions Ri for i = 1, ..., N, followed by N sums over the spins).
Concerning the general case of N particles with spin in 3d, if Ψ is interpreted as a probability amplitude, the probability density is:
and the probability that particle 1 is in region R1 with spin sz1 = m1 and particle 2 is in region R2 with spin sz2 = m2 etc. at time t is the integral of the probability density over these regions and spins:
In all cases, the normalization condition is:
The multidimensional Fourier transforms of the position or position-spin space wave functions yields momentum or momentum-spin space wave functions. The inner products of these momentum space wave functions are similar to the position cases above, with the corresponding normalization conditions.
Distinguishable and identical particles
In quantum mechanics there is a fundamental distinction between identical particles and distinguishable particles. For example, any two electrons are identical and fundamentally indistinguishable from each other; the laws of physics make it impossible to "stamp an identification number" on a certain electron to keep track of it.[14] This translates to a requirement on the wave function for a system of N identical particles all of common spin s; the wave function of the system is either totally symmetric or totally antisymmetric in all the positions of the particles:[15]
where the + sign occurs if the particles are all bosons (s = 0, 1, 2,...), and − sign if they are all fermions (s = 1/2, 3/2,...). For N identical particles there is no such thing as "mixed symmetry": the wave function cannot be symmetric for some of the particles and antisymmetric for others. Notice the physical interchange of particles corresponds to mathematically switching arguments in the wave function.
The antisymmetry feature of fermionic wave functions leads to the Pauli principle. Generally, bosonic and fermionic symmetry requirements are the manifestation of particle statistics and are present in other quantum state formalisms.
For N distinguishable particles (all the particles are different, no two are identical), the wave function is non-symmetric (neither symmetric nor antisymmetric).
Units of the wave function
Even though wave functions are complex numbers, both the real and imaginary parts each have the same units (the imaginary unit i is a number without unit). The units of ψ depend on the number of particles the wave function describes, and the number of spatial or momentum dimensions of the system. In general, for N particles with positions r1, r2, ..., rN in n spatial dimensions, the normalization conditions require ψ to have units of [length]−Nn/2. The square root of the length unit is removed when one finds |ψ|2, which has units of [length]−Nn.
In momentum space, length is replaced by momentum, and the units are [momentum]−Nn/2.
These results are true for particles with or without spin, since for particles with spin, the summations are over dimensionless spin quantum numbers.
Wave functions as elements of an abstract vector space
The set of all possible wave functions (at any given time) forms an abstract mathematical vector space. This vector space is infinite-dimensional, because there is no finite set of functions which can be added together in various combinations to create every possible function. Specifically, the entire wave function is treated as a single abstract vector:
where |Ψ⟩ is a "ket" (a vector) written in bra–ket notation. As always, the state vector for the system is solved from the Schrödinger equation (or other dynamical pictures of quantum mechanics):
The statement that "wave functions form an abstract vector space" means that it is possible multiply wave functions by complex numbers and add together different wave functions in a coherent superposition. If |ψ⟩ and |ϕ⟩ are two states in the vector space, and a and b are two complex numbers, then the linear combination
(subject to normalization) is also in the same vector space. The dual vectors are denoted as "bras", ⟨Ψ|, which do not live in the same space as |Ψ⟩, but instead the dual space:
where * denotes complex conjugate.
The inner product of two wave functions |Ψ1⟩ and |Ψ2⟩ can be defined by
For these reasons, wave functions are elements of a Hilbert space. See the quantum state article for more explanation of the Hilbert space formalism and its consequences to quantum physics.
There are several advantages to understanding wave functions as elements of an abstract vector space:
- All the powerful tools of linear algebra can be used to manipulate and understand wave functions. For example:
- Linear algebra explains how a vector space can be given a basis, and then any vector in the vector space can be expressed in this basis. This explains the relationship between a wave function in position space and a wave function in momentum space, and suggests that there are other possibilities too.
- Bra–ket notation can be used to manipulate wave functions.
- The idea that quantum states are vectors in an abstract vector space (technically, a complex projective Hilbert space) is completely general in all aspects of quantum mechanics and quantum field theory, whereas the idea that quantum states are complex-valued "wave" functions of space is only true in certain situations.
Following is a summary of the bra–ket formalism applied to wave functions, with general discrete or continuous bases.
Discrete and continuous bases
A Hilbert space with a discrete basis |εi⟩ for i = 1, 2...n is orthonormal if the inner product of all pairs of basis kets are given by the Kronecker delta:
Orthonormal bases are convenient to work with because the inner product of two vectors have simple expressions. A wave function |Ψ⟩ expressed in this discrete basis of the Hilbert space, and the corresponding bra in the dual space, are respectively given by:
where the complex numbers
are the components of the vector. The column vector is a useful representation in terms of matrices. The entire vector |Ψ⟩ is independent of the basis, but the components depend on the basis. If a change of basis is made, the components of the vector must also change to compensate.
A Hilbert space with a continuous basis { |ε⟩ } is orthonormal if the inner product of all pairs of basis kets are given by the Dirac delta function:
As with the discrete bases, a symbol ε is used in the basis states, two common notations are |ε⟩ and sometimes |Ψε⟩. A particular basis ket may be subscripted |ε0⟩ ≡ |Ψε0⟩ or primed |ε′⟩ ≡ |Ψε′⟩, or simply given another symbol in place of ε.
While discrete basis vectors are summed over a discrete index, continuous basis vectors are integrated over a continuous index (a variable of a function). In what follows, all integrals are with respect to the real-valued basis variable ε (not complex-valued), over the required range. Usually this is just the real line or subsets of it. The state |Ψ⟩ in the continuous basis of the Hilbert space, with the corresponding bra in the dual space, are respectively given by:[16]
where the components are the complex-valued functions
of a real variable ε.
Completeness conditions
The completeness conditions (also called closure relations) are
for the discrete and continuous orthonormal bases, respectively. An orthonormal set of kets form bases if and only if they satisfy these relations.[16] In each case, the equality to unity means this is an identity operator; its action on any state leaves it unchanged. Multiplying any state on the right of these gives the representation of the state |Ψ⟩ in the basis. The inner product of a first state |Ψ1⟩}} with a second |Ψ2⟩ can also be obtained by multiplying |Ψ1⟩ on the left and |Ψ2⟩ on the right of the relevant completeness condition.
Inner product
Physically, the nature of the inner product is dependent on the basis in use, because the basis is chosen to reflect the quantum state of the system.
If |Ψ1⟩ is a state in the above basis with components c1, c2, ..., cn and |Ψ2⟩ is another state in the same basis with components z1, z2, ..., zn, the inner product is the complex number:
If |Ψ1⟩ is a state in the above continuous basis with components Ψ1(ε′), and |Ψ2⟩ is another state in the same basis with components Ψ2(ε), the inner product is the complex number:
where the integrals are taken over all ε and ε′.
The square of the norm (magnitude) of the state vector |Ψ⟩ is given by the inner product of |Ψ⟩ with itself, a real number:
for the discrete and continuous bases, respectively. Each say the projection of a complex probability amplitude onto itself is real. If |Ψ⟩ is normalized, these expressions would be unity. If the state is not normalized, then dividing by its magnitude normalizes the state to:
Normalized components and probabilities
For the discrete basis, projecting the normalized state |ΨN⟩ onto a particular state the system may collapse to, |εq⟩, gives the complex number;
so the modulus squared of this gives a real number;
In the Copenhagen interpretation, this is the probability of state |εq⟩ occurring.
In the continuous basis, the projection of the normalized state onto some particular basis |ε′⟩ is a complex-valued function;
so the squared modulus is a real-valued function
In the Copenhagen interpretation, this function is the probability density function of measuring the observable ε′, so integrating this with respect to ε′ between a ≤ ε′ ≤ b gives:
the probability of finding the system with ε′ between ε′ = a and ε′ = b.
Wave function collapse
The physical meaning of the components of |Ψ⟩ is given by the wave function collapse postulate, also known as Wave function collapse. If the observable(s) ε (momentum and/or spin, position and/or spin, etc.) corresponding to states |εi⟩ has distinct and definite values, λi, and a measurement of that variable is performed on a system in the state |Ψ⟩ then the probability of measuring λi is |⟨εi|Ψ⟩|2. If the measurement yields λi, the system "collapses" to the state |εi⟩, irreversibly and instantaneously.
Time dependence
In the Schrödinger picture, the states evolve in time, so the time dependence is placed in |Ψ⟩ according to:[17]
for discrete bases, or
for continuous bases. However, in the Heisenberg picture the states |Ψ⟩ are constant in time and time dependence is placed in the Heisenberg operators, so |Ψ⟩ is not written as |Ψ(t)⟩.
Position representations
State space for one spin-0 particle in 1d
For a spinless particle in one spatial dimension (the x-axis or real line), the state |Ψ⟩ can be expanded in terms of a continuum of basis states; |x⟩, also written |Ψx⟩, corresponding to the set of all position coordinates x. The completeness condition for this basis is
and the orthogonality relation is
The state |Ψ⟩ is expressed by:
in which the "wave function" described as a function is a component of the complex state vector.
The inner product as stated at the beginning of this article is:
If the particle is confined to a region R (a subset of the x-axis), the integrals in the inner product and completeness condition would be integrals over R.
State space (other cases)
The previous example can be extended to more particles in higher dimensions, and include spin.
For one spinless particle in 3d, the basis states are |r⟩ and any state vector |Ψ⟩ in this space is expressed in terms of the basis vectors as |r⟩:
with components:
For N spinless particles in 3d, the basis states are |r1, ..., rN⟩. This is the tensor product of the one-particle position bases |r1⟩, |r2⟩, ..., |rN⟩, each of which spans the separate one-particle Hilbert spaces, so |r1, ..., rN⟩ are the basis states for the tensor product of the one-particle Hilbert spaces (the Hilbert space for the composite many particle system). Any state vector |Ψ⟩ in this space is
with components:
For one particle with spin in 3d, the basis states are |r, sz⟩, the tensor product of the position basis |r⟩ and spin basis |sz⟩, which exists in a new space from the spin space and position space alone. Any state |Ψ⟩ in this space is:
with components:
For N particles with spin in 3d, the basis states are |r1, ..., rN, sz 1, ..., sz N⟩, the tensor product of the position basis |r1, ..., rN⟩ and spin basis |sz 1, ..., sz N⟩, which exists in a new space from the spin space and position space alone. Any state in this space is:
with components:
If the particles are restricted to regions of position space, then the integrals in the completeness relations are taken over those regions, rather than the entire coordinate space. For the general case of many particles with spin in 3d, if particle 1 is in region R1, particle 2 is in region R2, and so on, the state in this position-spin representation is:
The orthogonality relation for this basis is:
and the inner product of |Ψ1⟩ and |Ψ2⟩ is:
Momentum space wave functions are similar, using the momentum vectors of the particles as continuous bases, namely |p⟩, |p1, p2, ..., pN⟩, etc.
Ontology
Whether the wave function really exists, and what it represents, are major questions in the interpretation of quantum mechanics. Many famous physicists of a previous generation puzzled over this problem, such as Schrödinger, Einstein and Bohr. Some advocate formulations or variants of the Copenhagen interpretation (e.g. Bohr, Wigner and von Neumann) while others, such as Wheeler or Jaynes, take the more classical approach[18] and regard the wave function as representing information in the mind of the observer, i.e. a measure of our knowledge of reality. Some, including Schrödinger, Einstein, Bohm and Everett and others, argued that the wave function must have an objective, physical existence. The latter argument is consistent with the fact that whenever two observers both think that a system is in a pure quantum state, they will always agree on exactly what state it is in (but this may not be true if one or both of them thinks the system is in a mixed state).[19] For more on this topic, see Interpretations of quantum mechanics.
Examples
Free particle
A free particle in 3d with wave vector k has a wave function
Particle in a box
A particle is restricted to a 1D region between x = 0 and x = L; its wave function is:
To normalize the wave function we need to find the value of the arbitrary constant A; solved from
From Ψ, we have |Ψ|2 = A2, so the integral becomes;
Solving this equation gives A = 1/√L, so the normalized wave function in the box is;
One-dimensional quantum tunnelling
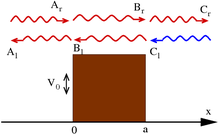
One of most prominent features of the wave mechanics is a possibility for a particle to reach a location with a prohibitive (in classical mechanics) force potential. In the one-dimensional case of particles with energy less than in the square potential
the steady-state solutions to the wave equation have the form (for some constants )
Note that these wave functions are not normalized; see scattering theory for discussion.
The standard interpretation of this is as a stream of particles being fired at the step from the left (the direction of negative x): setting Ar = 1 corresponds to firing particles singly; the terms containing Ar and Cr signify motion to the right, while Al and Cl – to the left. Under this beam interpretation, put Cl = 0 since no particles are coming from the right. By applying the continuity of wave functions and their derivatives at the boundaries, it is hence possible to determine the constants above.
Other
Some examples of wave functions for specific applications include:
See also
- Boson
- Double-slit experiment
- Faraday wave
- Fermion
- Schrödinger equation
- Wave function collapse
- Wave packet
References
- ^ R.G. Lerner, G.L. Trigg (1991). Encyclopaedia of Physics (2nd ed.). VHC Publishers. pp. 1223–1229. ISBN 0-89573-752-3.
- ^ Hanle, P.A. (December 1977), "Erwin Schrodinger's Reaction to Louis de Broglie's Thesis on the Quantum Theory.", Isis, 68 (4): 606–609, doi:10.1086/351880
- ^ Physics for Scientists and Engineers – with Modern Physics (6th Edition), P. A. Tipler, G. Mosca, Freeman, 2008, ISBN 0-7167-8964-7
- ^ Sears' and Zemansky's University Physics, Young and Freedman (12th edition), Pearson Ed. & Addison-Wesley Inc., 2008, ISBN 978-0-321-50130-1
- ^ a b c Quanta: A handbook of concepts, P.W. Atkins, Oxford University Press, 1974, ISBN 0-19-855493-1
- ^ Particle Physics (3rd Edition), B.R. Martin, G. Shaw, Manchester Physics Series, John Wiley & Sons, 2008, ISBN 978-0-470-03294-7
- ^ Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles (2nd Edition), R. Resnick, R. Eisberg, John Wiley & Sons, 1985, ISBN 978-0-471-87373-0
- ^ A.I.M Rae (2008). Quantum Mechanics. Vol. 2 (5th ed.). Taylor & Francis Group. ISBN 1-5848-89705.
- ^ P. W. Atkins (1974). Quanta: A Handbook of Concepts. p. 258. ISBN 0-19-855494-X.
- ^ Griffiths. Introduction to Quantum Mechanics (1st ed.). p. 107.
- ^ E. Abers (2004). Quantum Mechanics. Pearson Ed., Addison Wesley, Prentice Hall Inc. p. 115. ISBN 978-0-13-146100-0.
- ^ N. Zettili. Quantum Mechanics: Concepts and Applications (2nd ed.). p. 300. ISBN 978-0-470-02679-3.
- ^ N. Zettili. Quantum Mechanics: Concepts and Applications (2nd ed.). p. 468. ISBN 978-0-470-02679-3.
- ^ Griffiths, p. 179 of the first edition
- ^ N. Zettili. Quantum Mechanics: Concepts and Applications (2nd ed.). p. 463. ISBN 978-0-470-02679-3.
- ^ a b Y. Peleg, R. Pnini, E. Zaarur, E. Hecht. Quantum Mechanics. Schaum's Outlines (2nd ed.). pp. 64–65. ISBN 978-0-07-162358-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Y. Peleg, R. Pnini, E. Zaarur, E. Hecht (2010). Quantum mechanics. Schaum's outlines (2nd ed.). McGraw Hill. pp. 68–69. ISBN 978-0-07-162358-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ E. T. Jaynes. Probability Theory: The Logic of Science, Cambridge University Press (2003),
- ^ Pusey, Matthew F. (14 November 2011). "The quantum state cannot be interpreted statistically". arXiv.org: arxiv:1111.3328v1.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
2.Quantum Mechanics (Non-Relativistic Theory), L.D. Landau and E.M. Lifshitz, ISBN 0-08-020940-8
Further reading
- Griffiths, David J. (2004). Introduction to Quantum Mechanics (2nd ed.). Prentice Hall. ISBN 0-13-111892-7.
- Yong-Ki Kim (September 2, 2000). "Practical Atomic Physics" (PDF). National Institute of Standards and Technology. Maryland: 1 (55 pages). Retrieved 2010-08-17.
- Polkinghorne, John (2002). Quantum Theory, A Very Short Introduction. Oxford University Press. ISBN 0-19-280252-6.