Erythrosine: Difference between revisions
move part to regulations, US-specific |
→Regulation and prevalence: The sentence was confusing gramatically, but also very time sensitive, without any indication as to when it might stop being true. The sources did not support it. |
||
| Line 61: | Line 61: | ||
While erythrosine is commonly used in many countries of the world, it is less commonly used in the United States because [[Allura Red AC]] (Red #40) is generally used instead. Erythrosine can be used in colored food and ingested drugs in the US without any restriction; however, its use is banned in cosmetics and [[topical]] drugs.<ref>{{cite web | url=https://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm106626.htm | title=Color Additive Status List | work=Food & Drug Administration | date=December 2015 | access-date=May 16, 2016}}</ref> The [[Lake pigment|lake]] variant is also banned from use in the United States. |
While erythrosine is commonly used in many countries of the world, it is less commonly used in the United States because [[Allura Red AC]] (Red #40) is generally used instead. Erythrosine can be used in colored food and ingested drugs in the US without any restriction; however, its use is banned in cosmetics and [[topical]] drugs.<ref>{{cite web | url=https://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm106626.htm | title=Color Additive Status List | work=Food & Drug Administration | date=December 2015 | access-date=May 16, 2016}}</ref> The [[Lake pigment|lake]] variant is also banned from use in the United States. |
||
The [[European Food Safety Authority]] |
The [[European Food Safety Authority]] only allows erythrosine in processed cherries<ref>{{cite journal |last1=EFSA |title=Scientific Opinion on the re-evaluation of Erythrosine (E 127) as a food additive |journal=EFSA Journal |date=2011 |volume=9 |issue=1 |page=1854 |doi=10.2903/j.efsa.2011.1854|author1-link=EFSA }} |
||
Erythrosine is exclusively authorised for use in cocktail and candied cherries, and Bigarreaux cherries</ref> and pet foods.<ref>{{cite journal |last1=EFSA |title=Update of the Scientific Opinion on the safety and efficacy of erythrosine in feed for cats, dogs, reptiles and ornamental fish |journal=EFSA Journal |date=2015 |volume=13 |issue=9 |page=4233 |doi=10.2903/j.efsa.2015.4233|author1-link=EFSA |doi-access=free }}</ref><ref>{{cite journal |last1=EFSA|title=Safety of erythrosine for ornamental fish |journal=EFSA Journal |date=2019 |volume=17 |issue=5 |page=5699 |doi=10.2903/j.efsa.2019.5699|pmid=32626322 | pmc=7009114 |author1-link=EFSA }}</ref> |
Erythrosine is exclusively authorised for use in cocktail and candied cherries, and Bigarreaux cherries</ref> and pet foods.<ref>{{cite journal |last1=EFSA |title=Update of the Scientific Opinion on the safety and efficacy of erythrosine in feed for cats, dogs, reptiles and ornamental fish |journal=EFSA Journal |date=2015 |volume=13 |issue=9 |page=4233 |doi=10.2903/j.efsa.2015.4233|author1-link=EFSA |doi-access=free }}</ref><ref>{{cite journal |last1=EFSA|title=Safety of erythrosine for ornamental fish |journal=EFSA Journal |date=2019 |volume=17 |issue=5 |page=5699 |doi=10.2903/j.efsa.2019.5699|pmid=32626322 | pmc=7009114 |author1-link=EFSA }}</ref> |
||
Revision as of 00:56, 11 October 2023
| |
| Names | |
|---|---|
| IUPAC name
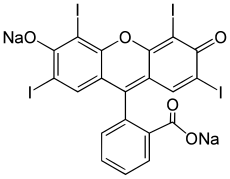
2-(6-Hydroxy-2,4,5,7-tetraiodo-3-oxo-xanthen-9-yl)benzoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.036.390 |
| E number | E127 (colours) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H6I4Na2O5 | |
| Molar mass | 879.86 g/mol |
| Melting point | 142 to 144 °C (288 to 291 °F; 415 to 417 K)[1] |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Erythrosine, also known as Red No. 3, is an organoiodine compound, specifically a derivative of fluorone. It is a pink dye which is primarily used for food coloring.[2] It is the disodium salt of 2,4,5,7-tetraiodofluorescein. Its maximum absorbance is at 530 nm[3] in an aqueous solution, and it is subject to photodegradation.
Uses
It is used as a:
- food coloring[4]
- printing ink[5]
- biological stain[6]
- dental plaque disclosing agent[7]
- radiopaque medium[6]
- sensitizer for orthochromatic photographic films
- Visible light photoredox catalyst[8][9]
Erythrosine is commonly used in sweets such as some candies and popsicles, and even more widely used in cake-decorating gels. It is also used to color pistachio shells.[10][failed verification][11][better source needed] As a food additive, it has the E number E127.
Regulation and prevalence
While erythrosine is commonly used in many countries of the world, it is less commonly used in the United States because Allura Red AC (Red #40) is generally used instead. Erythrosine can be used in colored food and ingested drugs in the US without any restriction; however, its use is banned in cosmetics and topical drugs.[12] The lake variant is also banned from use in the United States.
The European Food Safety Authority only allows erythrosine in processed cherries[13] and pet foods.[14][15]
United States
As a result of efforts begun in the 1970s, in 1990 the U.S. Food and Drug Administration (FDA) had instituted a partial ban on erythrosine, citing research that high doses have been found to cause cancer in rats.[16] A 1990 study concluded that "chronic erythrosine ingestion may promote thyroid tumor formation in rats via chronic stimulation of the thyroid by TSH." with 4% of total daily dietary intake consisting of erythrosine B.[17] A series of toxicology tests combined with a review of other reported studies concluded that erythrosine is non-genotoxic and any increase in tumors is caused by a non-genotoxic mechanism.[18]
In June 2008, the Center for Science in the Public Interest (CSPI) petitioned the FDA for a complete ban on erythrosine in the United States,[19] but the FDA has not taken any further action.
As of May 2023, the U.S. state of New York is considering banning the use of Red Dye No. 3 in foods (it was already banned from cosmetics as of 1990) because it has been shown to cause cancer in animals and due to claims that it, and other synthetic food dyes, may contribute to child behavioral problems such as hyperactivity.[20] California plans to ban Red Dye No. 3's usage in food starting in 2027, following a bill signed into law in October 2023.[21]
Synonyms
Erythrosine B; Erythrosin B; Acid Red 51; C.I. 45430; FD & C Red No.3; E127; 2',4',5',7'-Tetraiodo-3',6'-dihydroxy-spiro[3H-isobenzofuran-1,9'-xanthen]-3-one disodium salt; Tetraiodofluorescein Sodium Salt; Calcoid Erythrosine N; 2,4,5,7-Tetraiodo-3,6-dihydroxyxanthene-9-spiro-1'-3H-isobenzofuran-3'-one disodium salt; 2',4',5',7'-Tetraiodofluorescein, disodium salt; C.I.Food Red 14; Aizen Erythrosine; Tetraiodifluorescein, disodium salt; Spiro[isobenzofuran- 1(3H),9'-[9H]xanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt.[22][23]
Classification
It is listed under the following number systems:
- FD&C Red No. 3
- E number E127 (Food Red 14)
- Color Index no. 45430 (Acid Red 51)
- Bureau of Indian Standards No. 1697
References
- ^ "Erythrosine B product description". The Chemical Book.
- ^ Lyday PA (2005). "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- ^ Lancashire RJ. "Food Color Additives". Department of Chemistry, University of the West Indies. Archived from the original on 2007-01-28. Retrieved 2007-01-29.
- ^ Chequer FM, Venâncio VP, Bianchi ML, Antunes LM (October 2012). "Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells". Food and Chemical Toxicology. 50 (10): 3447–51. doi:10.1016/j.fct.2012.07.042. PMID 22847138.
- ^ Gurr E (2012). Synthetic Dyes in Biology, Medicine And Chemistry. elsevier. pp. 197, 198.
- ^ a b Dictionary of Analytical Reagents. CRC Press. 1993. p. 474. ISBN 978-0-412-35150-1.
- ^ Wood S, Metcalf D, Devine D, Robinson C (April 2006). "Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms". The Journal of Antimicrobial Chemotherapy. 57 (4): 680–4. doi:10.1093/jac/dkl021. PMID 16464894.
- ^ en:Photoredox_catalysis, oldid 915440777[circular reference]
- ^ Rogers DA, Brown RG, Brandeburg ZC, Ko EY, Hopkins MD, LeBlanc G, Lamar AA (October 2018). "N-Bromosuccinimide". ACS Omega. 3 (10): 12868–12877. doi:10.1021/acsomega.8b02320. PMC 6644467. PMID 31458011.
- ^ Cantor S (April 1997). "Kernels of Truth: Nuts". Food Product Design. Weeks Publishing Company. Archived from the original on 2007-07-23. Retrieved 2008-09-06.
- ^ Blue Diamond Ultra Premium Blend Mixed Nuts, distributed by Diamond Foods, Inc. Stockton, CA
- ^ "Color Additive Status List". Food & Drug Administration. December 2015. Retrieved May 16, 2016.
- ^ EFSA (2011). "Scientific Opinion on the re-evaluation of Erythrosine (E 127) as a food additive". EFSA Journal. 9 (1): 1854. doi:10.2903/j.efsa.2011.1854. Erythrosine is exclusively authorised for use in cocktail and candied cherries, and Bigarreaux cherries
- ^ EFSA (2015). "Update of the Scientific Opinion on the safety and efficacy of erythrosine in feed for cats, dogs, reptiles and ornamental fish". EFSA Journal. 13 (9): 4233. doi:10.2903/j.efsa.2015.4233.
- ^ EFSA (2019). "Safety of erythrosine for ornamental fish". EFSA Journal. 17 (5): 5699. doi:10.2903/j.efsa.2019.5699. PMC 7009114. PMID 32626322.
- ^ FDA: Red Dye's Reluctant Regulator; Partial Ban Points to Limitations of 30-Year-Old Delaney Clause, The Washington Post, February 7, 1990
- ^ Jennings AS, Schwartz SL, Balter NJ, Gardner D, Witorsch RJ (May 1990). "Effects of oral erythrosine (2',4',5',7'-tetraiodofluorescein) on the pituitary-thyroid axis in rats". Toxicology and Applied Pharmacology. 103 (3): 549–56. doi:10.1016/0041-008x(90)90327-q. PMID 2160137.
- ^ Lin GH, Brusick DJ (July 1986). "Mutagenicity studies on FD&C red No.3". Mutagenesis. 1 (4): 253–9. doi:10.1093/mutage/1.4.253. PMID 2457780.
- ^ "FDA Urged To Ban Some Food Dyes". CBS News. June 3, 2008. Archived from the original on 2012-08-27. Retrieved 2022-11-14.
- ^ Two States Have Proposed Bans on Common Food Additives Linked to Health Concerns by Dana G. Smith, April 13, 2023 on the New York Times website. Last access 5/23/2023.
- ^ "California becomes first US state to ban 4 potentially harmful chemicals in food". CNN. October 10, 2023.
- ^ Erythrosin B Archived 2010-03-17 at the Wayback Machine, University of South Carolina
- ^ Erythrosine, chemicalland21.com

