Ionic bonding: Difference between revisions
m Reverted edits by 147.31.184.122 to last revision by Decltype (HG) |
|||
| Line 32: | Line 32: | ||
Ionic substances in solution conduct electricity because the ions are free to move and carry the electrical charge from the anode to the cathode.<br /> |
Ionic substances in solution conduct electricity because the ions are free to move and carry the electrical charge from the anode to the cathode.<br /> |
||
Ionic substances conduct electricity when molten for the same reason i.e. that ions are free to move.<br /> |
Ionic substances conduct electricity when molten for the same reason i.e. that ions are free to move.<br /> |
||
Some ionic compounds conduct electricity when solid, this is due to migration of ions under the influence of an electric field. (see [[Fast ion conductor]]) |
Some ionic compounds conduct electricity when solid, this is due to migration of ions under the influence of an electric field. (see [[Fast ion conductor]])Also its very use full in some ways |
||
== Common anions and cations== |
== Common anions and cations== |
||
Revision as of 14:34, 16 April 2009
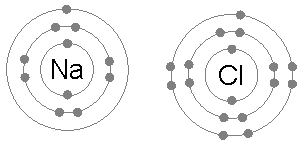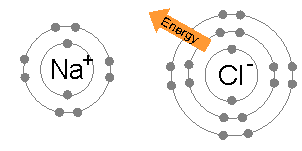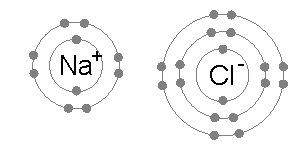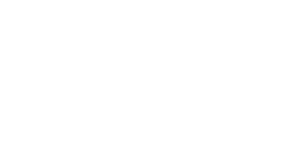
An ionic bond is a type of chemical bond that involves a metal and a non-metal ion (or polyatomic ions such as ammonium) through electrostatic attraction. In short, it is a bond formed by the attraction between two oppositely charged ions. The metal donates one or more electrons, forming a positively charged ion or cation with a stable electron configuration. These electrons then enter the non metal, causing it to form a negatively charged ion or anion which also has a stable electron configuration. The electrostatic attraction between the oppositely charged ions causes them to come together and form a bond.
For example, common table salt is Sodium Chloride. When sodium (Na) and chlorine (Cl) are combined, the sodium atoms each lose an electron, forming a cation (Na+), and the chlorine atoms each gain an electron to form an anion (Cl-). These ions are then attracted to each other in a 1:1 ratio to form sodium chloride (NaCl).
- Na + Cl → Na+ + Cl− → NaCl

The removal of electrons from the atoms is endothermic and causes the ions to have a higher energy. There may also be energy changes associated with breaking of existing bonds or the addition of more than one electron to form anions. However, the attraction of the ions to each other lowers their energy.
Ionic bonding will occur only if the overall energy change for the reaction is favourable – when the bonded atoms have a lower energy than the free ones. The larger the resulting energy change the stronger the bond. The low electronegativity of metals and high electronegativity of non-metals means that the energy change of the reaction is most favorable when metals lose electrons and non-metals gain electrons. An ionic bond doesn't need metal.
Pure ionic bonding is not known to exist. All ionic compounds have a degree of covalent bonding. The larger the difference in electronegativity between two atoms, the more ionic the bond. Ionic compounds conduct electricity when molten or in solution. They generally have a high melting point and tend to be soluble in water.
Ionic structure
Ionic compounds in the solid state form lattice structures, The two principal factors in determining the form of the lattice are the relative charges of the ions and their relative sizes. Some structures are adopted by a number of compounds, for example the rock salt, sodium chloride, structure is adopted by many alkaline earth halides and binary oxides such as MgO.
Strength of an ionic bond
For a solid crystalline ionic compound the enthalpy change in forming the solid from gaseous ions is termed the lattice energy. The experimental value for the lattice energy can be determined using the Born-Haber cycle. It can also be calculated using the Born-Landé equation as the sum of the electrostatic potential energy, calculated by summing interactions between cations and anions, and a short range repulsive potential energy term. The electrostatic potential can be expressed in terms of the inter-ionic separation and a constant (Madelung constant) that takes account of the geometry of the crystal. The Born-Landé equation gives a reasonable fit to the lattice energy of e.g. sodium chloride where the calculated value is −756 kJ/mol which compares to −787 kJ/mol using the Born-Haber cycle.[1]
Polarization effects
Ions in crystal lattices of purely ionic compounds are spherical; however, if the positive ion is small and/or highly charged, it will distort the electron cloud of the negative ion, an effect summarised in Fajans' rules. This polarization of the negative ion leads to a build-up of extra charge density between the two nuclei, i.e., to partial covalency. Larger negative ions are more easily polarized, but the effect is usually only important when positive ions with charges of 3+ (e.g., Al3+) are involved. However, 2+ ions (Be2+) or even 1+ (Li+) show some polarizing power because their sizes are so small (e.g., LiI is ionic but has some covalent bonding present). Note that this is not the ionic polarization effect which refers to displacement of ions in the lattice due to the application of an electric field.
Ionic versus covalent bonds
In an ionic bond, the atoms are bound by attraction of opposite ions, whereas, in a covalent bond, atoms are bound by sharing electrons. In covalent bonding, the molecular geometry around each atom is determined by VSEPR rules, whereas, in ionic materials, the geometry follows maximum packing rules.
Electrical conductivity
Ionic substances in solution conduct electricity because the ions are free to move and carry the electrical charge from the anode to the cathode.
Ionic substances conduct electricity when molten for the same reason i.e. that ions are free to move.
Some ionic compounds conduct electricity when solid, this is due to migration of ions under the influence of an electric field. (see Fast ion conductor)Also its very use full in some ways
Common anions and cations
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
External links
References
- ^ David Arthur Johnson, Metals and Chemical Change,Open University, Royal Society of Chemistry, 2002,ISBN 0854046658



