Umbilical cord: Difference between revisions
Epididymus10 (talk | contribs) No edit summary |
|||
| Line 1: | Line 1: | ||
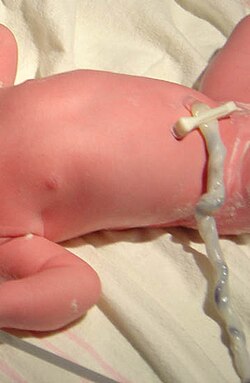
[[Image:Umbilicalcord.jpg|thumb|250px|right|Umbilical cord of a three-minute-old child. A medical clamp has been applied.]] |
[[Image:Umbilicalcord.jpg|thumb|250px|right|Umbilical cord of a three-minute-old child. A medical clamp has been applied.]] |
||
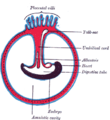
In placental [[mammal]]s, the '''umbilical cord''' (also called the '''birth cord''' or '''funiculus umbilicalis''') is the connecting cord from the developing [[embryo]] or [[fetus]] to the [[placenta]]. During [[prenatal development]], the umbilical cord comes from the same [[zygote]] as the fetus and (in humans) normally contains two [[arteries]] (the [[umbilical arteries]]) and one [[vein]] (the [[umbilical vein]]), buried within [[Wharton's jelly]]. The umbilical vein supplies the fetus with [[oxygen]]ated, [[nutrient]]-rich [[blood]] from the [[placenta]].<!-- |
In placental [[mammal]]s, the '''umbilical cord''' (also called the '''birth cord''' or '''funiculus umbilicalis''') is the connecting cord from the developing [[embryo]] or [[fetus]] to the [[placenta]]. During [[prenatal development]], the umbilical cord comes from the same [[zygote]] as the fetus and (in humans) normally contains two [[arteries]] (the [[umbilical arteries]]) and one [[vein]] (the [[umbilical vein]]), buried within [si[Wharton's jelly]]. The umbilical vein supplies the fetus with [[oxygen]]ated, [[nutrient]]-rich [[blood]] from the [[placenta]].<!-- |
||
****************** |
****************** |
||
| Line 68: | Line 68: | ||
[[Image:Umbilicalstump.jpg|thumb|250px|right|The cord stump of a seven-day-old baby]] |
[[Image:Umbilicalstump.jpg|thumb|250px|right|The cord stump of a seven-day-old baby]] |
||
General [[hospital]]-based [[obstetrics|obstetric]] practice introduces artificial clamping as early as 1 minute after the birth of the child. In [[birthing center]]s, this may be delayed by 5 minutes or more, or omitted entirely. Clamping is followed by cutting of the cord, which is painless due to the lack of any [[nerve]]s. The cord is extremely tough, like thick [[ |
General [[hospital]]-based [[obstetrics|obstetric]] practice introduces artificial clamping as early as 1 minute after the birth of the child. In [[birthing center]]s, this may be delayed by 5 minutes or more, or omitted entirely. Clamping is followed by cutting of the cord, which is painless due to the lack of any [[nerve]]s. The cord is extremely tough, like thick [[tendon]], and so cutting it requires a suitably sharp instrument. Provided that umbilical severance occurs after the cord has stopped pulsing (5-20 minutes after birth), there is ordinarily no significant loss of either venous or arterial blood while cutting the cord. |
||
There are umbilical cord clamps which combine the cord clamps with the knife. These clamps are safer and faster, allowing one to first apply the cord clamp and then cut the umbilical cord. After the cord is clamped and cut, the newborn wears a plastic clip on the navel area until the compressed region of the cord has dried and sealed sufficiently. The remaining umbilical stub remains for up to 7–10 days as it dries and then falls off. |
There are umbilical cord clamps which combine the cord clamps with the knife. These clamps are safer and faster, allowing one to first apply the cord clamp and then cut the umbilical cord. After the cord is clamped and cut, the newborn wears a plastic clip on the navel area until the compressed region of the cord has dried and sealed sufficiently. The remaining umbilical stub remains for up to 7–10 days as it dries and then falls off. |
||
Revision as of 02:15, 6 May 2010
In placental mammals, the umbilical cord (also called the birth cord or funiculus umbilicalis) is the connecting cord from the developing embryo or fetus to the placenta. During prenatal development, the umbilical cord comes from the same zygote as the fetus and (in humans) normally contains two arteries (the umbilical arteries) and one vein (the umbilical vein), buried within [si[Wharton's jelly]]. The umbilical vein supplies the fetus with oxygenated, nutrient-rich blood from the placenta. Conversely, the umbilical arteries return the deoxygenated, nutrient-depleted blood.
Physiology in humans
Development and composition
The umbilical cord develops from and contains remnants of the yolk sac and allantois (and is therefore derived from the same zygote as the foetus). It forms by the fifth week of fetal development, replacing the yolk sac as the source of nutrients for the foetus.[1] The cord is not directly connected to the mother's circulatory system, but instead joins the placenta, which transfers materials to and from the mother's blood without allowing direct mixing. The umbilical cord in a full term neonate is usually about 50 centimetres (20 in) long and about 2 centimetres (0.75 in) diameter. This diameter decreases rapidly within the placenta. The fully-patent umbilical artery has two main layers: an outer layer consisting of circularly arranged smooth muscle cells and an inner layer which shows rather irregularly and loosely arranged cells embedded in abundant ground substance staining metachromatic.[2] The smooth muscle cells of the layer are rather poorly differentiated, contain only a few tiny myofilaments and are thereby unlikely to contribute actively to the process of postnatal closure.[2]
The umbilical cord is composed of Wharton's jelly, a gelatinous substance made largely from mucopolysaccharides. It contains one vein, which carries oxygenated, nutrient-rich blood to the fetus and two arteries that carry deoxygenated, nutrient depleted blood away. Occasionally, only two vessels (one vein and one artery) are present in the umbilical cord. This is sometimes related to fetal abnormalities, but it may also occur without accompanying problems.
It is unusual for a vein to carry oxygenated blood, and for arteries to carry deoxygenated blood (the only other examples being the pulmonary veins and arteries, connecting the lungs to the heart). However, this naming convention reflects the fact that the umbilical vein carries blood towards the fetus's heart, while the umbilical arteries carry blood away.
Connection to foetal circulatory system
The umbilical cord enters the foetus via the abdomen, at the point which (after separation) will become the umbilicus (or navel). Within the foetus, the umbilical vein continues towards the transverse fissure of the liver, where it splits into two. One of these branches joins with the hepatic portal vein (connecting to its left branch), which carries blood into the liver. The second branch (known as the ductus venosus) allows the majority of the incoming blood (approximately 80%) to bypass the liver and flow via the left hepatic vein into the inferior vena cava, which carries blood towards the heart. The two umbilical arteries branch from the internal iliac arteries, and pass on either side of the urinary bladder before joining the umbilical cord.
Physiological postnatal occlusion
In absence of external interventions, the umbilical cord occludes physiologically shortly after birth, explained both by a swelling and collapse of Wharton's jelly in response to a reduction in temperature and by vasoconstriction of the blood vessels by smooth muscle contraction. In effect, a natural clamp is created, halting the flow of blood. If left to proceed naturally, this physiological clamping will take as little as five minutes and up to 20[3] minutes. In water birth in a warm waterbirth tub, where the temperature of the water may be equal to inside the body, normal pulsation can be 5 minutes and longer.[4]
Closure of the umbilical artery by vasoconstriction consists of multiple constrictions which increase in number and degree with time. There are segments of dilatations with trapped uncoagulated blood between the constrictionst before complete occlusion.[5] Both the partial constrictions and the ultimate closure are mainly produced by muscle cells of the outer circular layer.[2] In contrast, the inner layer seems to serve mainly as a plastic tissue which can easily be shifted in an axial direction and then folded into the narrowing lumen to complete the closure.[2] The vasoconstrictive occlusion appears to be mainly mediated by 5-hydroxytryptamine[6][7] and thromboxane A2[6]. The artery in cords of preterm infants contract relatively more to angiotensin II and arachidonic acid and are more sensitive to oxytocin than in term ones.[7] In contrast to the contribution of Wharton's jelly, cooling causes only temporary vasoconstriction.[7]
Within the child, the umbilical vein and ductus venosus close up, and degenerate into fibrous remnants known as the round ligament of the liver and the ligamentum venosum respectively. Part of each umbilical artery closes up (degenerating into what are known as the medial umbilical ligaments), while the remaining sections are retained as part of the circulatory system.
Problems and abnormalities

A number of abnormalities can affect the umbilical cord, which can cause problems that affect both mother and child:[8]
- Nuchal cord, when the umbilical cord becomes wrapped around the fetal neck[9]
- Single umbilical artery
- Umbilical cord prolapse
- Umbilical cord knot
- Umbilical cord entanglement
- Vasa previa
- Velamentous cord insertion
Medical protocols and procedures
Clamping and cutting


General hospital-based obstetric practice introduces artificial clamping as early as 1 minute after the birth of the child. In birthing centers, this may be delayed by 5 minutes or more, or omitted entirely. Clamping is followed by cutting of the cord, which is painless due to the lack of any nerves. The cord is extremely tough, like thick tendon, and so cutting it requires a suitably sharp instrument. Provided that umbilical severance occurs after the cord has stopped pulsing (5-20 minutes after birth), there is ordinarily no significant loss of either venous or arterial blood while cutting the cord.
There are umbilical cord clamps which combine the cord clamps with the knife. These clamps are safer and faster, allowing one to first apply the cord clamp and then cut the umbilical cord. After the cord is clamped and cut, the newborn wears a plastic clip on the navel area until the compressed region of the cord has dried and sealed sufficiently. The remaining umbilical stub remains for up to 7–10 days as it dries and then falls off.
Early versus delayed clamping
The health implications of early versus delayed cord clamping are receiving attention in medical journals.[10][11][12]
Delayed clamping may be supported by various health benefits: A recent analysis of attended home births over a 6-year period reported that none of the infants experienced adverse outcomes as a result of delayed cord clamping.[13] A meta-analysis[14] showed that delaying clamping of the umbilical cord in full-term neonates for a minimum of 2 minutes following birth is beneficial to the newborn in giving improved hematocrit, iron status as measured by ferritin concentration and stored iron, as well as a reduction in the risk of anemia (relative risk, 0.53; 95% CI, 0.40-0.70).[14] The decreased was also found in a study from 2008.[13] However, a Cochrane Review from 2008 showed that, although there is higher hemoglobin level at 2 months, this effect did not persist beyond 6 months of age.[15]
Negative effects of delayed cord clamping include an increased risk of polycythemia. Still, this condition appeared to be benign in studies.[14] The 2008 Cochrane review found that infants whose cord clamping occurred later than 60 seconds after birth had a statistically higher risk of neonatal jaundice requiring phototherapy. [15] Conversely, a recent randomized, controlled trial noted in the 2008 Examination of the Newborn & Neonatal Health compared the timing of cord clamping on the newborn venous hematocrit and reported an increase in anemia in the infants whose cords were clamped immediately.
Delayed clamping is not recommended for health care providers as a solution to cases where the newborn is not breathing well and needs resuscitation. Rather, the recommendation is instead to immediately clamp and cut the cord and perform cardiopulmonary resuscitation.[16] The umbilical cord pulsating is not a guarantee that the baby is receiving enough oxygen.[17]
Umbilical nonseverance
Some parents choose to omit cord severance entirely, a practice called "lotus birth" or umbilical nonseverance. The entire intact umbilical cord is allowed to dry like a sinew, which then separates naturally (typically on the 3rd day after birth), falling off and leaving a healed umbilicus. [18]
Umbilical cord catheterization
As the umbilical vein is directly connected to the central circulation, it can be used as a route for placement of a venous catheter for infusion and medication. The umbilical vein catheter is a reliable alternative to percutaneous peripheral or central venous catheters or intraosseous canulas and may be employed in resuscitation or intensive care of the newborn.
Storage of cord blood
Recently, it has been discovered that the blood within the umbilical cord, known as cord blood, is a rich and readily available source of primitive, undifferentiated stem cells (of type CD34-positive and CD38-negative). These cord blood cells can be used for bone marrow transplant.
Some parents have chosen to have this blood diverted from the baby's umbilical blood transfer through early cord clamping and cutting, to freeze for long-term (and costly) storage at a cord blood bank should the child ever require the cord blood stem cells (for example, to replace bone marrow destroyed when treating leukemia). This practice is controversial, with critics asserting that early cord blood withdrawal at the time of birth actually increases the likelihood of childhood disease, due to the high volume of blood taken (an average of 108ml) in relation to the baby's total supply (typically 300ml).[13] The Royal College of Obstetricians and Gynaecologists stated in 2006 that "there is still insufficient evidence to recommend directed commercial cord blood collection and stem-cell storage in low-risk families".
The American Academy of Pediatrics has stated that cord blood banking for self-use should be discouraged (as most conditions requiring the use of stem cells will already exist in the cord blood), while banking for general use should be encouraged.[19] In the future, cord blood-derived embryonic-like stem cells (CBEs) may be banked and matched with other patients, much like blood and transplanted tissues. The use of CBEs could potentially eliminate the ethical difficulties associated with embryonic stem cells (ESCs).[20]
While the American Academy of Pediatrics discourages private banking except in the case of existing medical need, it also says that information about the potential benefits and limitations of cord blood banking and transplantation should be provided so that parents can make an informed decision.
Cord blood education is also supported by legislators at the federal and state levels. In 2005, the National Academy of Sciences published an Institute of Medicine (IoM) report which recommended that expectant parents be given a balanced perspective on their options for cord blood banking. In response to their constituents, state legislators across the country are introducing legislation intended to help inform physicians and expectant parents on the options for donating, discarding or banking lifesaving newborn stem cells. Currently 17 states, covering two-thirds of U.S. births, have enacted legislation recommended by the IoM guidelines.
Research in this area that has the potential to revolutionize medicine is advancing rapidly and it is difficult for professional medical societies, and other resources that expectant parents turn to for information, to keep pace.
Physicians and researchers are making significant progress evaluating the safety and efficacy of umbilical cord blood stem cells for therapeutic uses far beyond cancers and blood disorders. The use of cord blood stem cells in treating conditions such as brain injury [21] and Type 1 Diabetes [22] is already being studied in humans, and earlier stage research is being conducted for treatments of stroke [23] [24], and hearing loss. [25]
The fundamental differences between private and public cord blood banking should be noted. Cord blood stored with private banks is reserved for use of the donor child only. In contrast, cord blood stored in public banks is accessible by anyone with a closely matching tissue type. The terms public and private do not necessarily indicate the funding source, but rather the availability of use.
The utilization of cord blood from public banks is rising rapidly. Currently it is used in place of a bone marrow transplant in the treatment of blood disorders such as leukemia, with donations released for transplant through one registry, Netcord, passing 9000. This is usually when the patient cannot find a matching bone marrow donor. It is this "extension" of the potential donor pool which has driven the expansion of public banks.
Private banks which collect for specific individuals store on the premise of future technologies and uses of cord blood. While this is a valid reason for private donation, it must be remembered that for many diseases such as leukemia, it is actually preferable to not use your own cord blood. This is due to the fact that the disease may be in latent form in your own cord blood, as well as a graft-versus-tumor effect.
The umbilical cord in other mammals
Anatomy
The umbilical cord in some mammals contains two distinct umbilical veins, rather than just one (as is the case for humans). Examples include cows and sheep.[26]
Cord disposal
In some animals, the mother will gnaw through the cord, thus separating the placenta from the offspring. It (along with the placenta) is often eaten by the mother, to provide nourishment and to dispose of tissues that would otherwise attract scavengers or predators. In chimpanzees, the mother focuses no attention on umbilical severance, instead nursing her baby with cord, placenta, and all, until the cord dries and separates within a day of birth, at which time the cord is discarded. (This was first documented by zoologists in the wild in 1974.[27])
Other uses for the term "umbilical cord"
The term "umbilical cord" or just "umbilical" has also come to be used for other cords with similar functions, such as the hose connecting a surface-supplied diver to his surface supply of air and/or heating, or a space-suited astronaut to his spacecraft. Engineers sometimes use the term to describe a complex or critical cable connecting a component, especially when composed of bundles of conductors of different colors, thickness and types, terminating in a single multi-contact disconnect.
The phrase "cutting the umbilical cord" is used metaphorically to describe a child's breaking away from the parental home, or any person severing a dependency on another person.
Additional images
-
Diagram illustrating a later stage in the development of the umbilical cord.
-
Fetus of about eight weeks, enclosed in the amnion. Magnified a little over two diameters
-
Sectional plan of the gravid uterus in the third and fourth month.
-
Fetus in utero, between fifth and sixth months.
-
Scheme of placental circulation.
-
Human embryo with heart and anterior body-wall removed to show the sinus venosus and its tributaries.
-
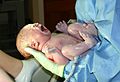
Newborn child, seconds after birth. The umbilical cord has not yet been cut.
-
Newborn child, soon after birth by Caesarian section.
-
Newborn child and mother, postpartum Umbilical Nonseverance.
-
Newborn child with cord attached, soon after Home birth.
See also
References
- ^ The Umbilical Cord
- ^ a b c d Meyer WW, Rumpelt HJ, Yao AC, Lind J (1978). "Structure and closure mechanism of the human umbilical artery". Eur. J. Pediatr. 128 (4): 247–59. PMID 668732.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Twenty-one frequently asked questions/answers concerning birthing procedures and vaccines D. Young and G.S. Goldman/Medical Veritas 5 (2008). In turn citing: Gunther M. The transfer of blood between baby and placenta in the minutes after birth. Lancet, 1957 Jun 22;272(6982):1277-1280
- ^ Common Questions about Lotus Birth Retrieved on Jan 10, 2009
- ^ Yao AC, Lind J, Lu T (1977). "Closure of the human umbilical artery: a physiological demonstration of Burton's theory". Eur. J. Obstet. Gynecol. Reprod. Biol. 7 (6): 365–8. PMID 264063.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Quan A, Leung SW, Lao TT, Man RY (2003). "5-hydroxytryptamine and thromboxane A2 as physiologic mediators of human umbilical artery closure". J. Soc. Gynecol. Investig. 10 (8): 490–5. PMID 14662162.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c White RP (1989). "Pharmacodynamic study of maturation and closure of human umbilical arteries". Am. J. Obstet. Gynecol. 160 (1): 229–37. PMID 2912087.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Umbilical Cord Complications: eMedicine Obstetrics and Gynecology". Retrieved 2010-01-24.
- ^ "Fetus or Newborn Problems: Labor and Delivery Complications: Merck Manual Home Edition". Retrieved 2010-03-27.
- ^ Hohmann M. (1985). "Early or late cord clamping? A question of optimal time" (Article in German). Wiener Klinische Wochenschrift, 97(11):497-500. PMID 4013344.
- ^ Mercer J.S., B.R. Vohr, M.M. McGrath, J.F. Padbury, M. Wallach, W. Oh (2006). "Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late-onset sepsis: a randomized, controlled trial." Pediatrics, 117(4):1235-42. PMID 16585320.
- ^ Hutton E.K., E.S. Hassan (2007). "Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials." Journal of the American Medical Association, 297(11):1257-58. PMID 17374818.
- ^ a b c Examination of the Newborn & Neonatal Health: A Multidimensional Approach, p. 116-117
- ^ a b c Hutton EK, Hassan ES (2007). "Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials". JAMA. 297 (11): 1241–52. doi:10.1001/jama.297.11.1241. PMID 17374818.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b "Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes." Cochrane Database Syst Rev. 2008; (2):CD004074
- ^ Military Obstetrics & Gynecology > Delivery of the Baby The Brookside Associates Medical Education Division. Retrieved on Jan 10, 2009
- ^ Waterbirth International > Waterbirth FAQ Retrieved on Jan 10, 2009
- ^ Crowther S (2006). "Lotus birth: leaving the cord alone." The Practising Midwife, 9(6):12-14. PMID 16830839.
- ^ American Academy of Pediatrics. "Cord Blood Banking for Potential Future Transplantation".
- ^ "Cord blood yields 'ethical' embryonic stem cells.", Coghlin A. New Scientist, August 18, 2005. Accessed June 25, 2007.
- ^ Cord Blood for Neonatal Hypoxic-Ischemic Encephalopathy, Autologous Cord Blood Cells for Hypoxic Ischemic Encephalopathy Study 1. Phase I Study of Feasibility and Safety
- ^ Haller MJ; et al. (2008). "Autologous umbilical cord blood infusion for type 1 diabetes". Exp. Hematol. 36 (6): 710–715. PMID 18358588.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Vendrame M; et al. (2006). "Cord blood rescues stroke-induced changes in splenocyte phenotype and function". Exp. Neurol. 199 (1): 191–200. PMID 16713598.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Vendrame M; et al. (2005). "Anti-inflammatory effects of human cord blood cells in a rat model of stroke". Stem Cells Dev. 14 (5): 595–604. PMID 16305344.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Revoltella RP; et al. (2008). "Cochlear repair by transplantation of human cord blood CD133+ cells to nod-scid mice made deaf with kanamycin and noise". Cell Transplant. 17 (6): 665–678. PMID 18819255.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Meat Hygiene y J. F. Gracey, D. S. Collins, Robert J. Huey. Page 32.
- ^ See In the Shadow of Man, by Jane Goodall.