Diethyl malonate: Difference between revisions
Change format for image |
|||
| Line 48: | Line 48: | ||
Diethyl malonate may be prepared by reacting the sodium salt of [[chloroacetic acid]] with [[sodium cyanide]], followed by [[base hydrolysis]] of the resultant [[nitrile]] to give the sodium salt malonic acid. [[Fischer esterification]] gives diethyl malonate: |
Diethyl malonate may be prepared by reacting the sodium salt of [[chloroacetic acid]] with [[sodium cyanide]], followed by [[base hydrolysis]] of the resultant [[nitrile]] to give the sodium salt malonic acid. [[Fischer esterification]] gives diethyl malonate: |
||
:[[File:Diethyl |
:[[File:Diethyl malonate synthesis.svg|500px]] |
||
==Reactions== |
==Reactions== |
||
Revision as of 18:32, 4 August 2010
| |
| |
| Names | |
|---|---|
| IUPAC name
1,3-diethyl propanedioate
| |
| Other names
diethyl malonate, DEM
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.006 |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C7H12O4 | |
| Molar mass | 160.17 g/mol |
| Appearance | colourless liquid |
| Density | 1.05 g/cm3, liquid |
| Melting point | −50 °C (223 K) |
| Boiling point | 199 °C (472 K) |
| negligible | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Harmful (X), Flammable (F) |
| Flash point | 200 °C |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
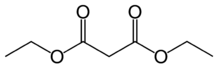
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6.
Structure and properties
Malonic acid is a rather simple dicarboxylic acid, with two carboxyl groups close together in its molecule. In forming diethyl malonate from malonic acid, the hydroxyl group (-OH) on both of the carboxyl groups is replaced by an ethoxy group (-OEt; -OCH2CH3). The methylene group (-CH2-) in the middle of the malonic part of the diethyl malonate molecule is neighboured by two carbonyl groups (-C(=O)-).[1]
The hydrogen atoms on the carbon adjacent to the carbonyl group in a molecule is slightly more acidic than hydrogen atoms on a carbon adjacent to alkyl groups. (This is known as the α position with respect to the carbonyl.) The hydrogen atoms on a carbon adjacent to two carbonyl groups are even more acidic because on the – the carbonyl groups helps stabilize the carbanion resulting from the removal of a proton from the methylene group between them.
Thus, this compound's conjugate base is stabilized by three resonance forms below:
Preparation
Diethyl malonate may be prepared by reacting the sodium salt of chloroacetic acid with sodium cyanide, followed by base hydrolysis of the resultant nitrile to give the sodium salt malonic acid. Fischer esterification gives diethyl malonate:
Reactions
Malonic ester synthesis
One of the principle uses of this compound is in the malonic ester synthesis. The carbanion (2) formed by reacting diethyl malonate (1) with a suitable base can be alkylated with a suitable electrophile. This alkylated 1,3-dicarbonyl compound (3) readily undergoes decarboxylation with loss of carbon dioxide, to give a substituted acetic acid (4):
Sodium ethoxide is preferred as the base. The use of aqueous sodium hydroxide may give the base hydrolysis products: sodium malonate and ethanol. In comparison, when sodium ethoxide is used, any nucleophilic attack at the carboxylate by the ethoxide will not give any side product; other alkoxide salts will cause scrambling by transesterification.
Other reactions
Like many other esters, this compound undergoes the Claisen ester condensations. The advantage of using this compound is that unwanted self-condensation reactions are avoided. Like other esters, this compound undergoes bromination at the alpha position.[2]
References
- ^ IR spectrum of Malonic acid
- ^ C. S. Palmer and P. W. McWherter. "Ethyl Bromoacetate". Organic Syntheses; Collected Volumes, vol. 1, p. 245.



