Potassium bitartrate: Difference between revisions
mNo edit summary |
|||
| Line 24: | Line 24: | ||
Potassium bitartrate [[crystal]]lises in wine casks during the [[fermentation (food)|fermentation]] of [[grape]] [[juice]], and can precipitate out of wine in bottle. The crystals will often form on the underside of a cork in wine bottles that have been stored at temperatures below 10 degrees Celsius (50 degrees Fahrenheit), and will seldom, if ever, dissolve naturally into the wine. |
Potassium bitartrate [[crystal]]lises in wine casks during the [[fermentation (food)|fermentation]] of [[grape]] [[juice]], and can precipitate out of wine in bottle. The crystals will often form on the underside of a cork in wine bottles that have been stored at temperatures below 10 degrees Celsius (50 degrees Fahrenheit), and will seldom, if ever, dissolve naturally into the wine. |
||
These crystals also precipitate out of fresh grape juice that has been chilled or allowed to stand for some time |
These crystals also precipitate out of fresh grape juice that has been chilled or allowed to stand for some time<ref>Lloyds Vinyard FAQs http://www.lloydsvineyard.com.au/faqs.php </ref>. To prevent crystals forming in home made grape jam or jelly, fresh grape juice should be chilled overnight to promote crystallization. The potassium bitartrate crystals are removed by filtering through two layers of cheesecloth; the filtered juice may then be made into jam or jelly<ref> National Center for Home Food Preservation http://www.uga.edu/nchfp/how/can_07/grape_jelly_powder.html </ref>. In some cases they adhere to the side of the chilled container, making filtering unnecessary. |
||
The crude form (known as '''beeswing''') is collected and purified to produce the white, odorless, [[acidic]] powder used for many culinary and other household purposes. |
The crude form (known as '''beeswing''') is collected and purified to produce the white, odorless, [[acidic]] powder used for many culinary and other household purposes. |
||
Revision as of 22:41, 17 August 2010
| |
| Names | |
|---|---|
| Other names
potassium hydrogen tartrate
cream of tartar potassium acid tartrate monopotassium tartrate | |
| Identifiers | |
| ECHA InfoCard | 100.011.609 |
| E number | E336 (antioxidants, ...) |
| Properties | |
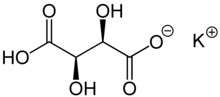
| KC4H5O6 | |
| Molar mass | 188.177 |
| Appearance | white crystalline powder |
| Density | 1.05 g/cm3 (solid) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium bitartrate, also known as potassium hydrogen tartrate, has formula KC4H5O6. It is a byproduct of winemaking. In cooking it is known as cream of tartar. It is a potassium acid salt of tartaric acid, a carboxylic acid (the other being potassium tartrate).
Occurrence
Potassium bitartrate crystallises in wine casks during the fermentation of grape juice, and can precipitate out of wine in bottle. The crystals will often form on the underside of a cork in wine bottles that have been stored at temperatures below 10 degrees Celsius (50 degrees Fahrenheit), and will seldom, if ever, dissolve naturally into the wine.
These crystals also precipitate out of fresh grape juice that has been chilled or allowed to stand for some time[1]. To prevent crystals forming in home made grape jam or jelly, fresh grape juice should be chilled overnight to promote crystallization. The potassium bitartrate crystals are removed by filtering through two layers of cheesecloth; the filtered juice may then be made into jam or jelly[2]. In some cases they adhere to the side of the chilled container, making filtering unnecessary.
The crude form (known as beeswing) is collected and purified to produce the white, odorless, acidic powder used for many culinary and other household purposes.
Applications
In food
In food, potassium bitartrate is used for:
- Stabilizing egg whites, increasing their heat tolerance and volume;
- Preventing sugar syrups from crystallising;
- Reducing discolouration of boiled vegetables;
- Frequent combination with baking soda (which needs an acid ingredient to activate it) in formulations of baking powder.
- Commonly used in combination with potassium chloride in sodium-free salt substitutes
- gingerbread house icing[citation needed].
A similar acid salt, sodium acid pyrophosphate, can be confused with cream of tartar because of their common function as a baking powder.
Household use
Potassium bitartrate can be used with white vinegar to make a paste-like cleaning agent.[citation needed] This mixture is sometimes mistakenly made with vinegar and sodium bicarbonate (baking soda), which actually react to neutralise each other, creating carbon dioxide and a sodium acetate solution.
It is a vital ingredient in Play-Doh[citation needed].
Chemistry
Potassium acid tartrate, also known as potassium hydrogen tartrate, is, according to NIST, used as a primary reference standard for a pH buffer. Using an excess of the salt in water, a saturated solution is created with a pH of 3.557 at 25 °C. Upon dissolution in water, potassium bitartrate will dissociate into acid tartrate, tartrate, and potassium ions. Thus, a saturated solution creates a buffer with standard pH. Before use as a standard, it is recommended that the solution be filtered or decanted between 22 °C and 28 °C.[3]
See also
- Tartrate
- Tartaric acid
- Potassium tartrate (K2C4H4O6)
References
- ^ Lloyds Vinyard FAQs http://www.lloydsvineyard.com.au/faqs.php
- ^ National Center for Home Food Preservation http://www.uga.edu/nchfp/how/can_07/grape_jelly_powder.html
- ^ Harris, Daniel C. (17 July 2006), Quantitative Chemical Analysis (7th ed.), New York: W. H. Freeman, ISBN 978-0716776949
External links
 This article incorporates text from a publication now in the public domain: Ward, Artemas (1911). The Grocer's Encyclopedia.
This article incorporates text from a publication now in the public domain: Ward, Artemas (1911). The Grocer's Encyclopedia. {{cite encyclopedia}}: Missing or empty|title=(help)
