Ferrite (iron): Difference between revisions
m Reverted edits by 117.199.194.71 (talk) to last revision by Wizard191 (HG) |
No edit summary |
||
| Line 9: | Line 9: | ||
[[Mild steel]] (carbon steel with up to about 0.2 wt% C) consist mostly of ferrite, with increasing amounts of [[pearlite]] (a fine lamellar structure of ferrite and [[cementite]]) as the carbon content is increased. Since [[bainite]] (shown as ledeburite on the diagram) and pearlite each have ferrite as a component, any iron-carbon alloy will contain some amount of ferrite if it is allowed to reach [[chemical equilibrium|equilibrium]] at room temperature. |
[[Mild steel]] (carbon steel with up to about 0.2 wt% C) consist mostly of ferrite, with increasing amounts of [[pearlite]] (a fine lamellar structure of ferrite and [[cementite]]) as the carbon content is increased. Since [[bainite]] (shown as ledeburite on the diagram) and pearlite each have ferrite as a component, any iron-carbon alloy will contain some amount of ferrite if it is allowed to reach [[chemical equilibrium|equilibrium]] at room temperature. |
||
In pure iron, ferrite is stable below {{convert|910|C|F|abbr=on}}. Above this temperature the [[face-centered cubic]] form of iron, [[austenite]] (gamma-iron) is stable. Above {{convert|1390|C|F|abbr=on}}, up to the [[melting point]] at {{convert|1539|C|F|abbr=on}}, the body-centred cubic crystal structure is again the more stable form of '''delta-ferrite''' ('''δ-Fe'''). |
In pure iron, ferrite is stable below {{convert|910|C|F|abbr=on}}. Above this temperature the [[face-centered cubic|face-centred cubic]] form of iron, [[austenite]] (gamma-iron) is stable. Above {{convert|1390|C|F|abbr=on}}, up to the [[melting point]] at {{convert|1539|C|F|abbr=on}}, the body-centred cubic crystal structure is again the more stable form of '''delta-ferrite''' ('''δ-Fe'''). |
||
Only a very small amount of [[carbon]] can be dissolved in ferrite; the maximum [[solubility]] is about 0.02 wt% at {{convert|723|C}} and 0.005% carbon at {{convert|0|C}}.<ref>{{harvnb|Smith|Hashemi|2006|p=363}}.</ref> This is because carbon dissolves in iron interstitially, with the carbon atoms being about twice the diameter of the interstitial "holes", so that each carbon atom is surrounded by a strong local [[strain field]]. Hence the [[enthalpy]] of mixing is positive (unfavourable), but the contribution of [[entropy]] to the [[Thermodynamic free energy|free energy]] of [[solution]] stabilises the structure for low carbon content. {{convert|723|C|F|abbr=on}} also is the minimum temperature at which iron-carbon austenite (0.8 wt% C) is stable; at this temperature there is a [[eutectoid]] reaction between ferrite, austenite and cementite. |
Only a very small amount of [[carbon]] can be dissolved in ferrite; the maximum [[solubility]] is about 0.02 wt% at {{convert|723|C}} and 0.005% carbon at {{convert|0|C}}.<ref>{{harvnb|Smith|Hashemi|2006|p=363}}.</ref> This is because carbon dissolves in iron interstitially, with the carbon atoms being about twice the diameter of the interstitial "holes", so that each carbon atom is surrounded by a strong local [[strain field]]. Hence the [[enthalpy]] of mixing is positive (unfavourable), but the contribution of [[entropy]] to the [[Thermodynamic free energy|free energy]] of [[solution]] stabilises the structure for low carbon content. {{convert|723|C|F|abbr=on}} also is the minimum temperature at which iron-carbon austenite (0.8 wt% C) is stable; at this temperature there is a [[eutectoid]] reaction between ferrite, austenite and cementite. |
||
Revision as of 15:56, 29 December 2010
| Steels |
|---|
 |
| Phases |
| Microstructures |
| Classes |
| Other iron-based materials |
This article needs additional citations for verification. (October 2008) |
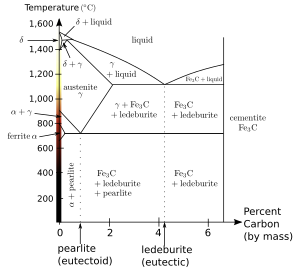
Ferrite or alpha iron (α-Fe) is a materials science term for iron, or a solid solution with iron as the main constituent, with a body centred cubic crystal structure. It is the component which gives steel and cast iron their magnetic properties, and is the classic example of a ferromagnetic material. Practically speaking, it can be considered pure iron.[1]
It has a strength of 280 N/mm2[citation needed] and a hardness of approximately 80 Brinell.[2]
Mild steel (carbon steel with up to about 0.2 wt% C) consist mostly of ferrite, with increasing amounts of pearlite (a fine lamellar structure of ferrite and cementite) as the carbon content is increased. Since bainite (shown as ledeburite on the diagram) and pearlite each have ferrite as a component, any iron-carbon alloy will contain some amount of ferrite if it is allowed to reach equilibrium at room temperature.
In pure iron, ferrite is stable below 910 °C (1,670 °F). Above this temperature the face-centred cubic form of iron, austenite (gamma-iron) is stable. Above 1,390 °C (2,530 °F), up to the melting point at 1,539 °C (2,802 °F), the body-centred cubic crystal structure is again the more stable form of delta-ferrite (δ-Fe).
Only a very small amount of carbon can be dissolved in ferrite; the maximum solubility is about 0.02 wt% at 723 °C (1,333 °F) and 0.005% carbon at 0 °C (32 °F).[3] This is because carbon dissolves in iron interstitially, with the carbon atoms being about twice the diameter of the interstitial "holes", so that each carbon atom is surrounded by a strong local strain field. Hence the enthalpy of mixing is positive (unfavourable), but the contribution of entropy to the free energy of solution stabilises the structure for low carbon content. 723 °C (1,333 °F) also is the minimum temperature at which iron-carbon austenite (0.8 wt% C) is stable; at this temperature there is a eutectoid reaction between ferrite, austenite and cementite.
See also
References
- ^ Maranian, Peter (2009), Reducing Brittle and Fatigue Failures in Steel Structures, New York: American Society of Civil Engineers, ISBN 9780784410677.
- ^ Structure of plain steel, retrieved 2008-10-21.
- ^ Smith & Hashemi 2006, p. 363.
Bibliography
- Smith, William F.; Hashemi, Javad (2006), Foundations of Materials Science and Engineering (4th ed.), McGraw-Hill, ISBN 0-07-295358-6.
