High-performance liquid chromatography: Difference between revisions
Hero of Ludi (talk | contribs) |
Hero of Ludi (talk | contribs) |
||
| Line 119: | Line 119: | ||
*Void volume is the amount of space in a column that is occupied by solvent. It is the space within the column that is outside of the column's internal packing material. Void volume is measured on a chromatagram as the first component peak detected, which is usually the solvent that was present in the sample mixture; ideally the sample solvent flows through the column without interacting with the column, but is still detectable as distinct from the HPLC solvent. The void volume is used as a correction factor other factors. |
*Void volume is the amount of space in a column that is occupied by solvent. It is the space within the column that is outside of the column's internal packing material. Void volume is measured on a chromatagram as the first component peak detected, which is usually the solvent that was present in the sample mixture; ideally the sample solvent flows through the column without interacting with the column, but is still detectable as distinct from the HPLC solvent. The void volume is used as a correction factor other factors. |
||
*Efficiency factor (''N'') practically measures how sharp component peaks on the chromatagram are, as ratio of the component peak's area ("retention time") relative to the width of the peaks at their widest point (at the baseline). Peaks that are tall, sharp, and relatively narrow indicate that separation method efficiently removed a component from a mixture; high efficiency. Efficiency is very dependent upon the HPLC column and the HPLC method used. Efficiency factor is synonymous with plate number, and the 'number of theoretical plates'. |
*Efficiency factor (''N'') practically measures how sharp component peaks on the chromatagram are, as ratio of the component peak's area ("retention time") relative to the width of the peaks at their widest point (at the baseline). Peaks that are tall, sharp, and relatively narrow indicate that separation method efficiently removed a component from a mixture; high efficiency. Efficiency is very dependent upon the HPLC column and the HPLC method used. Efficiency factor is synonymous with plate number, and the 'number of theoretical plates'. |
||
*Retention factor (''kappa prime'') measures how long a component of the mixture stuck to the column, by area under the curve in a chromatagram. This is corrected for by the void volume of the column. |
*Retention factor (''kappa prime'') measures how long a component of the mixture stuck to the column, by area under the curve in a chromatagram. Each chromatagram peak will have it's own retention factor (e.g. kappa-1 for the retention factor of the first peak). This is corrected for by the void volume of the column. |
||
*Separation factor (''alpha'') practically is a relative comparison how well two neighboring components of the mixture were separated (i.e. two neighboring bands on a chromatagram). This factor is defined in terms of the retention factor, and is also corrected for by the void volume of the column. The greater the alpha value is over 1.0, the better the separation, until about 2.0 beyond which an HPLC method is probably not needed for separation. |
*Separation factor (''alpha'') practically is a relative comparison how well two neighboring components of the mixture were separated (i.e. two neighboring bands on a chromatagram). This factor is defined in terms of the retention factor, and is also corrected for by the void volume of the column. The greater the alpha value is over 1.0, the better the separation, until about 2.0 beyond which an HPLC method is probably not needed for separation. |
||
Resolution equations relate the three factors such that high efficiency and separation factors improve the resolution of component peaks in a HPLC separation. |
Resolution equations relate the three factors such that high efficiency and separation factors improve the resolution of component peaks in a HPLC separation. |
||
Revision as of 04:01, 8 October 2012
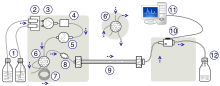
 A HPLC. From left to right: A pumping device generating a gradient of two different solvents, a steel enforced column and an apparatus for measuring the absorbance. | |
| Acronym | HPLC |
|---|---|
| Classification | Chromatography |
| Analytes | organic molecules biomolecules ions polymers |
| Other techniques | |
| Related | Chromatography Aqueous Normal Phase Chromatography Hydrophilic Interaction Chromatography Ion exchange chromatography Size exclusion chromatography Micellar liquid chromatography |
| Hyphenated | Liquid chromatography-mass spectrometry |

This article may be too technical for most readers to understand. (June 2010) |
High-performance liquid chromatography (sometimes referred to as high-pressure liquid chromatography), HPLC, is a chromatographic technique used to separate a mixture of compounds in analytical chemistry and biochemistry with the purpose of identifying, quantifying and purifying the individual components of the mixture. HPLC is also considered an instrumentation technique of analytical chemistry, instead of a gravitimetric technique. HPLC has many uses including medical (e.g. detecting vitamin D concentrations in blood serum), legal (e.g.detecting performance enhancement drugs in urine), research (e.g. purifying substances from a complex biological sample, or separating similar synthetic chemicals from each other), and manufacturing (e.g. pharmaceutical quality assurance).
HPLC relies on the pressure of mechanical pumps on a liquid solvent to load a sample mixture onto a separation column, in which the separation occurs. A HPLC separation column is filled with solid particles (e.g. silica, polymers, or sorbents), and the sample mixture is separated into compounds as it interacts with the column particles. HPLC separation is influenced by the liquid solvent’s condition (e.g. pressure, temperature), chemical interactions between the sample mixture and the liquid solvent (e.g. hydrophobicity, protonation, etc…), and chemical interactions between the sample mixture and the solid particles packed inside of the separation column (e.g. Ligand affinity, ion exchange, etc...).
HPLC is distinguished from ordinary liquid chromatography because the pressure of HPLC is relatively high, while ordinary liquid chromatography typically relies on the force of gravity to provide pressure. Due to the higher pressure separation conditions of HPLC, HPLC columns have relatively small internal diameter (e.g. 4.6 mm), are short (e.g. 25 mm), and packed more densely with smaller particles, which helps achieve finer separations of a sample mixture than ordinary liquid chromatography can. This gives HPLC superior resolving power when separating mixtures, which is why it is a popular chromatographic technique.
The schematic of an HPLC instrument typically includes a sampler by which the sample mixture is injected into the HPLC, one or more mechanical pumps for pushing liquid through a tubing system, a separation column, a digital analyte detector (e.g. a UV/Vis, or a photodiode array (PDA)) for qualitative or quantitative analysis of the separation, and a digital microprocessor for controlling the HPLC components (and user software). Many different types of columns are available, varying in size, and in the type (i.e. chemistry) of solid packed particle types available. Some models of mechanical pumps in a HPLC instrument can also mix multiple liquids together, and the recipe or gradient of those liquids can modify the chemical interactions that occur in HPLC’s column, and thereby modify the chemical separation of the mixture.
Operation
The sample to be separated and analyzed is introduced, in a discrete small volume, into the stream of mobile phase percolating through the column. The components of the sample move through the column at different velocities, which are functions of specific physical or chemical interactions with the stationary phase. The velocity of each component depends on its chemical nature, on the nature of the stationary phase (column) and on the composition of the mobile phase. The time at which a specific analyte elutes (emerges from the column) is called the retention time. The retention time measured under particular conditions is considered an identifying characteristic of a given analyte. The use of smaller particle size packing materials requires the use of higher operational pressure ("backpressure") and typically improves chromatographic resolution (i.e. the degree of separation between consecutive analytes emerging from the column). Common mobile phases used include any miscible combination of water with various organic solvents (the most common are acetonitrile and methanol). Some HPLC techniques use water free mobile phases (see Normal Phase HPLC below). The aqueous component of the mobile phase may contain buffers, acids (such as formic, phosphoric or trifluoroacetic acid) or salts to assist in the separation of the sample components. The composition of the mobile phase may be kept constant ("isocratic elution mode") or varied ("gradient elution mode") during the chromatographic analysis. Isocratic elution is typically effective in the separation of sample components that are not very dissimilar in their affinity for the stationary phase.
In gradient elution the composition of the mobile phase is varied typically from low to high eluting strength. The eluting strength of the mobile phase is reflected by analyte retention times with high eluting strength producing fast elution (=short retention times). A typical gradient profile in reversed phase chromatography might start at 5% acetonitrile (in water or aqueous buffer) and progress linearly to 95% acetonitrile over 5–25 minutes. Period of constant mobile phase composition may be part of any gradient profile. For example, the mobile phase composition may be kept constant at 5% acetonitrile for 1–3 min, followed by a linear change up to 95% acetonitrile.
The composition of the mobile phase depends on the intensity of interactions between analytes and stationary phase (e.g. hydrophobic interactions in reversed-phase HPLC). Depending on their affinity for the stationary and mobile phases analytes partition between the two during the separation process taking place in the column. This partitioning process is similar to that which occurs during a liquid-liquid extraction but is continuous, not step-wise. In this example, using a water/acetonitrile gradient, more hydrophobic components will elute (come off the column) late, once the mobile phase gets more concentrated in acetonitrile (i.e. in a mobile phase of higher eluting strength).
The choice of mobile phase components, additives (such as salts or acids) and gradient conditions depend on the nature of the column and sample components. Often a series of trial runs are performed with the sample in order to find the HPLC method which gives the best separation.
Types
Partition chromatography

Partition chromatography was the first kind of chromatography that chemists developed. The partition coefficient principle has been applied in paper chromatography, thin layer chromatography, gas phase and liquid-liquid applications. The 1952 Nobel Prize in chemistry was earned by Archer John Porter Martin and Richard Laurence Millington Synge for their development of the technique, which was used for their separation of amino acids. Partition chromatography uses a retained solvent, on the surface or within the grains or fibers of an "inert" solid supporting matrix as with paper chromatography; or takes advantage of some coulombic and/or hydrogen donor interaction with the solid support. Molecules equilibrate (partition) between a liquid stationary phase and the eluent. Known as Hydrophilic Interaction Chromatography (HILIC) in HPLC, this method separates analytes based on polar differences. HILIC most often uses a bonded polar stationary phase and water miscible, high organic concentration, mobile phases. Partition HPLC has been used historically on unbonded silica or alumina supports. Each works effectively for separating analytes by relative polar differences. HILIC bonded phases have the advantage of separating acidic, basic and neutral solutes in a single chromatogram.[1]
The polar analytes diffuse into a stationary water layer associated with the polar stationary phase and are thus retained. Retention strengths increase with increased analyte polarity, and the interaction between the polar analyte and the polar stationary phase (relative to the mobile phase) increases the elution time. The interaction strength depends on the functional groups in the analyte molecule which promote partitioning but can also include coulombic (electrostatic) interaction and hydrogen donor capability.
Use of more polar solvents in the mobile phase will decrease the retention time of the analytes, whereas more hydrophobic solvents tend to increase retention times.
Normal-phase chromatography
It was one of the first kinds of HPLC that chemists developed. Also known as normal-phase HPLC (NP-HPLC), or adsorption chromatography, this method separates analytes based on their affinity for a polar stationary surface such as silica, hence it is based on analyte ability to engage in polar interactions (such as hydrogen-bonding or dipole-dipole type of interactions) with the sorbent surface. NP-HPLC uses a non-polar, non-aqueous mobile phase, and works effectively for separating analytes readily soluble in non-polar solvents. The analyte associates with and is retained by the polar stationary phase. Adsorption strengths increase with increased analyte polarity. The interaction strength depends not only on the functional groups present in the structure of the analyte molecule, but also on steric factors. The affect of steric hindrance on interaction strength allows this method to resolve (separate) structural isomers.
The use of more polar solvents in the mobile phase will decrease the retention time of analytes, whereas more hydrophobic solvents tend to induce slower elution (increased retention times). Very polar solvents such as traces of water in the mobile phase tend to adsorb to the solid surface of the stationary phase forming a stationary bound (water) layer which is considered to play an active role in retention. This behavior is somewhat peculiar to normal phase chromatograhy because it is governed almost exclusively by an adsorptive mechanism (i.e. analytes interact with a solid surface rather than with the solvated layer of a ligand attached to the sorbent surface; see also reversed-phase HPLC below). Adsorption chromatography is still widely used for structural isomer separations in both column and thin-layer chromatography formats on activated (dried) silica or alumina supports.
Partition- and NP-HPLC fell out of favor in the 1970s with the development of reversed-phase HPLC because of poor reproducibility of retention times due to the presence of a water or protic organic solvent layer on the surface of the silica or alumina chromatographic media. This layer changes with any changes in the composition of the mobile phase (e.g. moisture level) causing drifting retention times.
Recently, partition chromatography has become popular again with the development of HILIC bonded phases which demonstrate improved reproducibility, and due to a better understanding of the range of usefulness of the technique.
Displacement chromatography
The basic principle of displacement chromatography is: A molecule with a high affinity for the chromatography matrix (the displacer) will compete effectively for binding sites, and thus displace all molecules with lesser affinities.[2] There are distinct differences between displacement and elution chromatography. In elution mode, substances typically emerge from a column in narrow, Gaussian peaks. Wide separation of peaks, preferably to baseline, is desired in order to achieve maximum purification. The speed at which any component of a mixture travels down the column in elution mode depends on many factors. But for two substances to travel at different speeds, and thereby be resolved, there must be substantial differences in some interaction between the biomolecules and the chromatography matrix. Operating parameters are adjusted to maximize the effect of this difference. In many cases, baseline separation of the peaks can be achieved only with gradient elution and low column loadings. Thus, two drawbacks to elution mode chromatography, especially at the preparative scale, are operational complexity, due to gradient solvent pumping, and low throughput, due to low column loadings. Displacement chromatography has advantages over elution chromatography in that components are resolved into consecutive zones of pure substances rather than “peaks”. Because the process takes advantage of the nonlinearity of the isotherms, a larger column feed can be separated on a given column with the purified components recovered at significantly higher concentration .
Reversed-phase chromatography (RPC)
Reversed phase HPLC (RP-HPLC) has a non-polar stationary phase and an aqueous, moderately polar mobile phase. One common stationary phase is a silica which has been surface-modified with RMe2SiCl, where R is a straight chain alkyl group such as C18H37 or C8H17. With such stationary phases, retention time is longer for molecules which are less polar, while polar molecules elute more readily (early in the analysis). An investigator can increase retention times by adding more water to the mobile phase; thereby making the affinity of the hydrophobic analyte for the hydrophobic stationary phase stronger relative to the now more hydrophilic mobile phase. Similarly, an investigator can decrease retention time by adding more organic solvent to the eluent. RP-HPLC is so commonly used that it is often incorrectly referred to as "HPLC" without further specification. The pharmaceutical industry regularly employs RP-HPLC to qualify drugs before their release.
RP-HPLC operates on the principle of hydrophobic interactions, which originates from the high symmetry in the dipolar water structure and plays the most important role in all processes in life science. RP-HPLC allows the measurement of these interactive forces. The binding of the analyte to the stationary phase is proportional to the contact surface area around the non-polar segment of the analyte molecule upon association with the ligand on the stationary phase. This solvophobic effect is dominated by the force of water for "cavity-reduction" around the analyte and the C18-chain versus the complex of both. The energy released in this process is proportional to the surface tension of the eluent (water: 7.3×10−6 J/cm², methanol: 2.2×10−6 J/cm²) and to the hydrophobic surface of the analyte and the ligand respectively. The retention can be decreased by adding a less polar solvent (methanol, acetonitrile) into the mobile phase to reduce the surface tension of water. Gradient elution uses this effect by automatically reducing the polarity and the surface tension of the aqueous mobile phase during the course of the analysis.
Structural properties of the analyte molecule play an important role in its retention characteristics. In general, an analyte with a larger hydrophobic surface area (C-H, C-C, and generally non-polar atomic bonds, such as S-S and others) is retained longer because it is non-interacting with the water structure. On the other hand, analytes with higher polar surface area (conferred by the presence of polar groups, such as -OH, -NH2, COO– or -NH3+ in their structure) are less retained as they are better integrated into water. Such interactions are subject to steric effects in that very large molecules may have only restricted access to the pores of the stationary phase, where the interactions with surface ligands (alkyl chains) take place. Such surface hindrance typically results in less retention.
Retention time increases with hydrophobic (non-polar) surface area. Branched chain compounds elute more rapidly than their corresponding linear isomers because the overall surface area is decreased. Similarly organic compounds with single C-C-bonds elute later than those with a C=C or C-C-triple bond, as the double or triple bond is shorter than a single C-C-bond.
Aside from mobile phase surface tension (organizational strength in eluent structure), other mobile phase modifiers can affect analyte retention. For example, the addition of inorganic salts causes a moderate linear increase in the surface tension of aqueous solutions (ca. 1.5×10−7 J/cm² per Mol for NaCl, 2.5×10−7 J/cm² per Mol for (NH4)2SO4), and because the entropy of the analyte-solvent interface is controlled by surface tension, the addition of salts tend to increase the retention time. This technique is used for mild separation and recovery of proteins and protection of their biological activity in protein analysis (hydrophobic interaction chromatography, HIC).
Another important factor is the mobile phase pH since it can change the hydrophobic character of the analyte. For this reason most methods use a buffering agent, such as sodium phosphate, to control the pH. Buffers serve multiple purposes: control of pH, neutralize the charge on the silica surface of the stationary phase and act as ion pairing agents to neutralize analyte charge. Ammonium formate is commonly added in mass spectrometry to improve detection of certain analytes by the formation of analyte-ammonium adducts. A volatile organic acid such as acetic acid, or most commonly formic acid, is often added to the mobile phase if mass spectrometry is used to analyze the column effluent. Trifluoroacetic acid is used infrequently in mass spectrometry applications due to its persistence in the detector and solvent delivery system, but can be effective in improving retention of analytes such as carboxylic acids in applications utilizing other detectors, as it is a fairly strong organic acid. The effects of acids and buffers vary by application but generally improve chromatographic resolution.
Reversed phase columns are quite difficult to damage compared with normal silica columns; however, many reversed phase columns consist of alkyl derivatized silica particles and should never be used with aqueous bases as these will destroy the underlying silica particle. They can be used with aqueous acid, but the column should not be exposed to the acid for too long, as it can corrode the metal parts of the HPLC equipment. RP-HPLC columns should be flushed with clean solvent after use to remove residual acids or buffers, and stored in an appropriate composition of solvent. The metal content of HPLC columns must be kept low if the best possible ability to separate substances is to be retained. A good test for the metal content of a column is to inject a sample which is a mixture of 2,2'- and 4,4'- bipyridine. Because the 2,2'-bipy can chelate the metal, the shape of the peak for the 2,2'-bipy will be distorted (tailed) when metal ions are present on the surface of the silica.[citation needed]..
Size-exclusion chromatography
Size-exclusion chromatography (SEC), also known as gel permeation chromatography or gel filtration chromatography, separates particles on the basis of molecular size (actually by a particle's Stokes radius). It is generally a low resolution chromatography and thus it is often reserved for the final, "polishing" step of a purification. It is also useful for determining the tertiary structure and quaternary structure of purified proteins. SEC is used primarily for the analysis of large molecules such as proteins or polymers. SEC works by trapping these smaller molecules in the pores of a particle. The larger molecules simply pass by the pores as they are too large to enter the pores. Larger molecules therefore flow through the column quicker than smaller molecules, that is, the smaller the molecule, the longer the retention time.
This technique is widely used for the molecular weight determination of polysaccharides. SEC is the official technique (suggested by European pharmacopeia) for the molecular weight comparison of different commercially available low-molecular weight heparins.
Ion-exchange chromatography
In ion-exchange chromatography (IC), retention is based on the attraction between solute ions and charged sites bound to the stationary phase. Solute ions of the same charge as the charged sites on the column are excluded from binding, while solute ions of the opposite charge of the charged sites of the column are retained on the column. Solute ions that are retained on the column can be eluted from the column by changing the solvent conditions (e.g. increasing the ion effect of the solvent system by increasing the salt concentration of the solution, increasing the column temperature, changing the pH of the solvent, etc...).
Types of ion exchangers include:
- Polystyrene resins – These allow cross linkage which increases the stability of the chain. Higher cross linkage reduces swerving, which increases the equilibration time and ultimately improves selectivity.
- Cellulose and dextran ion exchangers (gels) – These possess larger pore sizes and low charge densities making them suitable for protein separation.
- Controlled-pore glass or porous silica
In general, ion exchangers favor the binding of ions of higher charge and smaller radius.
An increase in counter ion (with respect to the functional groups in resins) concentration reduces the retention time. A decrease in pH reduces the retention time in cation exchange while an increase in pH reduces the retention time in anion exchange. By lowering the pH of the solvent in a cation exchange column, for instance, more hydrogen ions are available to compete for positions on the anionic stationary phase, thereby eluting weakly bound cations.
This form of chromatography is widely used in the following applications: water purification, preconcentration of trace components, ligand-exchange chromatography, ion-exchange chromatography of proteins, high-pH anion-exchange chromatography of carbohydrates and oligosaccharides, and others.
Bioaffinity chromatography
This chromatographic process relies on the property of biologically active substances to form stable, specific, and reversible complexes. The formation of these complexes involves the participation of common molecular forces such as the Van der Waals interaction, electrostatic interaction, dipole-dipole interaction, hydrophobic interaction, and the hydrogen bond. An efficient, biospecific bond is formed by a simultaneous and concerted action of several of these forces in the complementary binding sites.
Aqueous normal-phase chromatography
Aqueous normal-phase chromatography (ANP) is a chromatographic technique which encompasses the mobile phase region between reversed-phase chromatography (RP) and organic normal phase chromatography (ONP). This technique is used to achieve unique selectivity for hydrophilic compounds, showing normal phase elution using reversed-phase solvents. [citation needed]
Isocratic flow and gradient elution
A separation in which the mobile phase composition remains constant throughout the procedure is termed isocratic (meaning constant composition). The word was coined by Csaba Horvath who was one of the pioneers of HPLC.[citation needed],
The mobile phase composition does not have to remain constant. A separation in which the mobile phase composition is changed during the separation process is described as a gradient elution.[3] One example is a gradient starting at 10% methanol and ending at 90% methanol after 20 minutes. The two components of the mobile phase are typically termed "A" and "B"; A is the "weak" solvent which allows the solute to elute only slowly, while B is the "strong" solvent which rapidly elutes the solutes from the column. In reversed-phase chromatography, solvent A is often water or an aqueous buffer, while B is an organic solvent miscible with water, such as acetonitrile, methanol, THF, or isopropanol.
In isocratic elution, peak width increases with retention time linearly according to the equation for N, the number of theoretical plates. This leads to the disadvantage that late-eluting peaks get very flat and broad. Their shape and width may keep them from being recognized as peaks.
Gradient elution decreases the retention of the later-eluting components so that they elute faster, giving narrower (and taller) peaks for most components. This also improves the peak shape for tailed peaks, as the increasing concentration of the organic eluent pushes the tailing part of a peak forward. This also increases the peak height (the peak looks "sharper"), which is important in trace analysis. The gradient program may include sudden "step" increases in the percentage of the organic component, or different slopes at different times – all according to the desire for optimum separation in minimum time.
In isocratic elution, the selectivity does not change if the column dimensions (length and inner diameter) change – that is, the peaks elute in the same order. In gradient elution, the elution order may change as the dimensions or flow rate change.[citation needed]
The driving force in reversed phase chromatography originates in the high order of the water structure. The role of the organic component of the mobile phase is to reduce this high order and thus reduce the retarding strength of the aqueous component.
Parameters
Theoretical
HPLC separations have theoretical parameters and equations to describe the separation of components into signal peaks when detected by instrumentation such as by a UV detector or mass spectrometer. The parameters are practical in that they are not predicted, but measurements of HPLC chromatagrams. They are analogous to the calculation of retention factor for a thin layer chromatography (TLC) separation, but describes how well HPLC separates a mixture into two or more components that are detected as peaks (bands) on a chromatagrams. The HPLC parameters are the: efficiency factor(N), the retention factor (kappa prime), and the separation factor (alpha). Together the factors are variables in a resolution equation, which describes how well two components' peaks separated or overlapped each other. These parameters are mostly only used for describing HPLC reversed phase and HPLC normal phase separations, since those separations tend to be more subtle than other HPLC modes (e.g. ion exchange and size exclusion).
- Void volume is the amount of space in a column that is occupied by solvent. It is the space within the column that is outside of the column's internal packing material. Void volume is measured on a chromatagram as the first component peak detected, which is usually the solvent that was present in the sample mixture; ideally the sample solvent flows through the column without interacting with the column, but is still detectable as distinct from the HPLC solvent. The void volume is used as a correction factor other factors.
- Efficiency factor (N) practically measures how sharp component peaks on the chromatagram are, as ratio of the component peak's area ("retention time") relative to the width of the peaks at their widest point (at the baseline). Peaks that are tall, sharp, and relatively narrow indicate that separation method efficiently removed a component from a mixture; high efficiency. Efficiency is very dependent upon the HPLC column and the HPLC method used. Efficiency factor is synonymous with plate number, and the 'number of theoretical plates'.
- Retention factor (kappa prime) measures how long a component of the mixture stuck to the column, by area under the curve in a chromatagram. Each chromatagram peak will have it's own retention factor (e.g. kappa-1 for the retention factor of the first peak). This is corrected for by the void volume of the column.
- Separation factor (alpha) practically is a relative comparison how well two neighboring components of the mixture were separated (i.e. two neighboring bands on a chromatagram). This factor is defined in terms of the retention factor, and is also corrected for by the void volume of the column. The greater the alpha value is over 1.0, the better the separation, until about 2.0 beyond which an HPLC method is probably not needed for separation.
Resolution equations relate the three factors such that high efficiency and separation factors improve the resolution of component peaks in a HPLC separation.
Internal diameter
The internal diameter (ID) of an HPLC column is an important parameter that influences the detection sensitivity and separation selectivity in gradient elution. It also determines the quantity of analyte that can be loaded onto the column. Larger columns are usually seen in industrial applications, such as the purification of a drug product for later use. Low-ID columns have improved sensitivity and lower solvent consumption at the expense of loading capacity.
- Larger ID columns (over 10 mm) are used to purify usable amounts of material because of their large loading capacity.
- Analytical scale columns (4.6 mm) have been the most common type of columns, though smaller columns are rapidly gaining in popularity. They are used in traditional quantitative analysis of samples and often use a UV-Vis absorbance detector.
- Narrow-bore columns (1–2 mm) are used for applications when more sensitivity is desired either with special UV-vis detectors, fluorescence detection or with other detection methods like liquid chromatography-mass spectrometry
- Capillary columns (under 0.3 mm) are used almost exclusively with alternative detection means such as mass spectrometry. They are usually made from fused silica capillaries, rather than the stainless steel tubing that larger columns employ.
Particle size
Most traditional HPLC is performed with the stationary phase attached to the outside of small spherical silica particles (very small beads). These particles come in a variety of sizes with 5 m beads being the most common. Smaller particles generally provide more surface area and better separations, but the pressure required for optimum linear velocity increases by the inverse of the particle diameter squared.[4][5][6]
This means that changing to particles that are half as big, keeping the size of the column the same, will double the performance, but increase the required pressure by a factor of four. Larger particles are used in preparative HPLC (column diameters 5 cm up to >30 cm) and for non-HPLC applications such as solid-phase extraction.
Pore size
Many stationary phases are porous to provide greater surface area. Small pores provide greater surface area while larger pore size has better kinetics, especially for larger analytes. For example, a protein which is only slightly smaller than a pore might enter the pore but does not easily leave once inside.
Pump pressure
Pumps vary in pressure capacity, but their performance is measured on their ability to yield a consistent and reproducible flow rate. Pressure may reach as high as 40 MPa (6000 lbf/in2), or about 400 atmospheres. Modern HPLC systems have been improved to work at much higher pressures, and therefore are able to use much smaller particle sizes in the columns (<2 m). These "Ultra High Performance Liquid Chromatography" systems or RSLC/UHPLCs can work at up to 100 MPa (15,000 lbf/in²), or about 1000 atmospheres. The term "UPLC" is a trademark of the Waters Corporation, but is sometimes used to refer to the more general technique.
See also
References
- ^ Lindsay, S. ; Kealey, D. (1987). High performance liquid chromatography. Wiley.
{{cite book}}: CS1 maint: multiple names: authors list (link) from review Hung, L. B.; Parcher, J. F.; Shores, J. C.; Ward, E. H. (1988). "Theoretical and experimental foundation for surface-coverage programming in gas-solid chromatography with an adsorbable carrier gas". J. Am. Chem. Soc. 110 (11): 1090. doi:10.1021/ac00162a003. - ^ Displacement Chromatography. Sacheminc.com. Retrieved on 2011-06-07.
- ^ A recent book provides a comprehensive treatment of the theory of high-performance gradient chromatography: Lloyd R. Snyder and John W. Dolan (2006). High-Performance Gradient Elution: The Practical Application of the Linear-Solvent-Strength Model. Wiley Interscience. ISBN 0-471-70646-9.
{{cite book}}: Unknown parameter|unused_data=ignored (help) - ^ Majors, Ronald E.. (2010-09-07) Fast and Ultrafast HPLC on sub-2 μm Porous Particles — Where Do We Go From Here? – LC-GC Europe. Lcgceurope.com. Retrieved on 2011-06-07.
- ^ Xiang, Y. (2006). "Ultrahigh pressure liquid chromatography using elevated temperature". Journal of Chromatography A. 1104 (1–2): 198–202. doi:10.1016/j.chroma.2005.11.118. PMID 16376355.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Horváth, Cs. (1967). "Fast liquid chromatography. Investigation of operating parameters and the separation of nucleotides on pellicular ion exchangers". Analytical Chemistry. 39 (12): 1422–1428. doi:10.1021/ac60256a003. PMID 6073805.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
Further reading
- L. R. Snyder, J.J. Kirkland, and J. W. Dolan, Introduction to Modern Liquid Chromatography, John Wiley & Sons, New York, 2009.
- M.W. Dong, Modern HPLC for practicing scientists. Wiley, 2006.
- L. R. Snyder, J.J. Kirkland, and J. L. Glajch, Practical HPLC Method Development, John Wiley & Sons, New York, 1997.
- S. Ahuja and H. T. Rasmussen (ed), HPLC Method Development for Pharmaceuticals, Academic Press, 2007.
- S. Ahuja and M.W. Dong (ed), Handbook of Pharmaceutical Analysis by HPLC, Elsevier/Academic Press, 2005.
- Y. V. Kazakevich and R. LoBrutto (ed.), HPLC for Pharmaceutical Scientists, Wiley, 2007.
- U. D. Neue, HPLC Columns: Theory, Technology, and Practice, Wiley-VCH, New York, 1997.
- M. C. McMaster, HPLC, a practical user's guide, Wiley, 2007.

