Heat: Difference between revisions
→Heat transfer in engineering: Try to explain this. Pure flow of an adiabatic hot fluid, is not a transfer of heat. There is no cooling or heating, so there can be no heat transfer. See TALK |
No edit summary |
||
| Line 4: | Line 4: | ||
[[File:171879main LimbFlareJan12 lg.jpg|300px|thumb|right|Nuclear fusion in the [[Sun]] converts nuclear potential energy into available internal energy and keeps the temperature of the Sun very high. Consequently, heat is transported to Earth as [[electromagnetic radiation]]. This is the main source of energy for [[life]] on Earth.]] |
[[File:171879main LimbFlareJan12 lg.jpg|300px|thumb|right|Nuclear fusion in the [[Sun]] converts nuclear potential energy into available internal energy and keeps the temperature of the Sun very high. Consequently, heat is transported to Earth as [[electromagnetic radiation]]. This is the main source of energy for [[life]] on Earth.]] |
||
In [[physics]] and [[chemistry]], '''heat''' (or heat transfer or heat flow) is [[energy]] transferred from one body to another by thermal interaction.<ref>Reif, F. (1965), pp. 67, 73.</ref><ref name="Kittel and Kroemer 1980">Kittel, C. Kroemer, H. (1980). ''Thermal Physics'', second edition, W.H. Freeman, San Francisco, ISBN 0-7167-1088-9, p. 227.</ref> The transfer of energy can occur |
In [[physics]] and [[chemistry]], '''heat''' (or heat transfer or heat flow) is [[energy]] transferred from one body to another by thermal interaction.<ref>Reif, F. (1965), pp. 67, 73.</ref><ref name="Kittel and Kroemer 1980">Kittel, C. Kroemer, H. (1980). ''Thermal Physics'', second edition, W.H. Freeman, San Francisco, ISBN 0-7167-1088-9, p. 227.</ref> The transfer of energy can occur through two basic mechanisms: [[Thermal conduction|conduction]]<ref>{{harvnb|Guggenheim, E.A. (1949/1967)|page=8}}.</ref> and [[Thermal radiation | radiation]];<ref>Planck. M. (1914). ''The Theory of Heat Radiation'', a translation by Masius, M. of the second German edition, P. Blakiston's Son & Co., Philadelphia.</ref> or through mechanisms that combine mass and heat transfer such as [[Convective heat transfer|convection]]. Heat is not a property of a system or body, but instead is always associated with a process of some kind. |
||
Heat flow from a high to a low [[temperature]] body occurs spontaneously. This flow of energy can be harnessed and partly converted into useful work by means of a [[heat engine]]. The [[second law of thermodynamics]] prohibits heat flow directly from a low to a high temperature body, but with the aid of a [[heat pump]] external work can be used to transport [[internal energy]] indirectly from a low to a high temperature body. |
Heat flow from a high to a low [[temperature]] body occurs spontaneously. This flow of energy can be harnessed and partly converted into useful work by means of a [[heat engine]]. The [[second law of thermodynamics]] prohibits heat flow directly from a low to a high temperature body, but with the aid of a [[heat pump]] external work can be used to transport [[internal energy]] indirectly from a low to a high temperature body. |
||
Heat is a characteristic of [[Macroscopic scale|macroscopic]] processes and is described by [[thermodynamics]], but its origin and properties can be understood in terms of microscopic constituents using [[statistical mechanics]]. For instance, heat flow can occur when the rapidly vibrating molecules in a high temperature body transfer some of their energy (by direct contact, radiation exchange, or other mechanisms) to the more slowly vibrating molecules in a lower temperature body. |
Heat is a characteristic of [[Macroscopic scale|macroscopic]] processes and is described by [[thermodynamics]], but its origin and properties can be understood in terms of microscopic constituents using [[statistical mechanics]]. For instance, heat flow can occur when the rapidly vibrating molecules in a high temperature body transfer some of their energy (by direct contact, radiation exchange, or other mechanisms) to the more slowly vibrating molecules in a lower temperature body. |
||
Revision as of 01:04, 22 November 2012
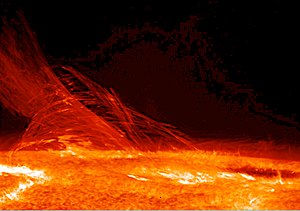
In physics and chemistry, heat (or heat transfer or heat flow) is energy transferred from one body to another by thermal interaction.[1][2] The transfer of energy can occur through two basic mechanisms: conduction[3] and radiation;[4] or through mechanisms that combine mass and heat transfer such as convection. Heat is not a property of a system or body, but instead is always associated with a process of some kind.
Heat flow from a high to a low temperature body occurs spontaneously. This flow of energy can be harnessed and partly converted into useful work by means of a heat engine. The second law of thermodynamics prohibits heat flow directly from a low to a high temperature body, but with the aid of a heat pump external work can be used to transport internal energy indirectly from a low to a high temperature body.
Heat is a characteristic of macroscopic processes and is described by thermodynamics, but its origin and properties can be understood in terms of microscopic constituents using statistical mechanics. For instance, heat flow can occur when the rapidly vibrating molecules in a high temperature body transfer some of their energy (by direct contact, radiation exchange, or other mechanisms) to the more slowly vibrating molecules in a lower temperature body.
The SI unit of heat is the joule. Heat can be measured by calorimetry,[5] or determined indirectly by calculations based on other quantities, relying for instance on the first law of thermodynamics. In physics, especially in calorimetry, and in meteorology, the concepts of latent heat and of sensible heat are used. Latent heat produces changes of state without temperature change, while sensible heat produces temperature change.
Overview

Heat in physics is defined as energy transferred by thermal interactions. Heat flows spontaneously from systems of higher temperature to systems of lower temperature. When two systems come into thermal contact, they exchange energy through the microscopic interactions of their particles. When the systems are at different temperatures, this entails spontaneous net flow of energy from the hotter to the cooler, so that the hotter decreases in temperature and the cooler increases in temperature. This will continue until their temperatures are equal. Then the net flow of energy has settled to zero, and the systems are said to be in a relation of thermal equilibrium. Spontaneous heat transfer is an irreversible process.
The first law of thermodynamics states that the internal energy of an isolated system is conserved. To change the internal energy of a system, energy must be transferred to or from the system. For a closed system, heat and work are the mechanisms by which energy can be transferred. For an open system, internal energy can be changed also by transfer of matter.[6] Work performed by a body is, by definition, an energy transfer from the body that is due to a change to external or mechanical parameters of the body, such as the volume, magnetization, and location of center of mass in a gravitational field.[2][7][8][9][10][11]
When a body is heated, its temperature and internal energy increase. This additional energy is stored as kinetic and potential energy of the atoms and molecules in the body. [12] Heat itself is not stored within a body. Like work, it exists only as energy in transit from one body to another or between a body and its surroundings.
Microscopic origin of heat
Heat is a macroscopic characteristic of systems, but like other thermodynamic quantities it has a fundamental origin in statistical mechanics -- the physics of the underlying microscopic degrees of freedom.
For example, the temperature of a gas is proportional to the kinetic energy of the motion of gas molecules. The average translational kinetic energy per unit of absolute temperature of a molecule of an ideal gas has the value of three halves of the Boltzmann constant.[13] Heat transfer between a low and high temperature gas brought into contact arises due to the exchange of kinetic and potential energy in molecular collisions. As more and more molecules undergo collisions, their kinetic energy equilibrates to an equilibrium distribution that corresponds to an intermediate temperature somewhere between the low and high initial temperatures of the two gases. An early and vague expression of this was by Francis Bacon.[14][15] Precise and detailed versions of it were developed in the nineteenth century.[16]
For solids, conduction of heat occurs through collective motions of microscopic particles, such as phonons, or through the motion of mobile particles like conduction band electrons. [17] As these excitations move around inside the solid and interact with it and each other, they transfer energy from higher to lower temperature regions, eventually leading to equilibration.
Definitions
Scottish physicist James Clerk Maxwell, in his 1871 classic Theory of Heat, was one of many who began to build on the already established idea that heat has something to do with matter in motion. This was the same idea put forth by Sir Benjamin Thompson in 1798, who said he was only following up on the work of many others. One of Maxwell's recommended books was Heat as a Mode of Motion, by John Tyndall. Maxwell outlined four stipulations for the definition of heat:
- It is something which may be transferred from one body to another, according to the second law of thermodynamics.
- It is a measurable quantity, and thus treated mathematically.
- It cannot be treated as a substance, because it may be transformed into something that is not a substance, e.g., mechanical work.
- Heat is one of the forms of energy.
Mechanisms of heat transfer
Referring to conduction, Partington writes: "If a hot body is brought in conducting contact with a cold body, the temperature of the hot body falls and that of the cold body rises, and it is said that a quantity of heat has passed from the hot body to the cold body."[18]
Referring to radiation, Maxwell writes: "In Radiation, the hotter body loses heat, and the colder body receives heat by means of a process occurring in some intervening medium which does not itself thereby become hot."[19]
From these empirically based ideas of heat, and from other empirical observations, the notions of internal energy and of entropy can be derived, so as to lead to the recognition of the first and second laws of thermodynamics.[20] This was the way of the historical pioneers of thermodynamics.[21][22]
Notation and units
As a form of energy heat has the unit joule (J) in the International System of Units (SI). However, in many applied fields in engineering the British Thermal Unit (BTU) and the calorie are often used. The standard unit for the rate of heat transferred is the watt (W), defined as joules per second.
The total amount of energy transferred as heat is conventionally written as Q for algebraic purposes. Heat released by a system into its surroundings is by convention a negative quantity (Q < 0); when a system absorbs heat from its surroundings, it is positive (Q > 0). Heat transfer rate, or heat flow per unit time, is denoted by . This should not be confused with a time derivative of a function of state (which can also be written with the dot notation) since heat is not a function of state. Heat flux is defined as rate of heat transfer per unit cross-sectional area, resulting in the unit watts per square metre.
Estimation of quantity of heat
The quantity of heat transferred by some process can either be directly measured, or determined indirectly through calculations based on other quantities.
Direct measurement is by calorimetry and is the primary empirical basis of the idea of quantity of heat transferred in a process. The transferred heat is measured by changes in a body of known properties, for example, temperature rise, change in volume or length, or phase change, such as melting of ice.[23][24]
Indirect estimations of quantity of heat transferred rely on the law of conservation of energy, and, in particular cases, on the first law of thermodynamics. Indirect estimation is the primary approach of many theoretical studies of quantity of heat transferred.[25][26][27]
Internal energy and enthalpy
In the case where the number of particles in the system is constant (closed systems), the first law of thermodynamics states that the differential change in internal energy dU of a system is given by an infinitesimal amount of heat δQ supplied to the system minus the infinitesimal amount of work δW exerted by the system:[note 1]
This can also be interpreted as that δQ makes contributions to the internal energy and to the work done by the system:
The work done by the system includes boundary work (when the system increases its volume against an external force, such as that exerted by a piston) and other work (e.g. shaft work performed by a compressor fan):
In this Section we will neglect the "other-work" contribution. The internal energy, U, is a state function. In cyclical processes, such as the operation of a heat engine, state functions return to their initial values after completing one cycle. Thus, the differential for the internal energy is an exact differential dU. The symbol for exact differentials is the lowercase letter d.
In contrast, neither Q nor W represents the state of the system. Thus, infinitesimal amounts of heat and work are inexact differentials, denoted by δQ and δW, respectively. The lowercase Greek letter delta, δ, is the symbol for inexact differentials. The integral of any inexact differential over the time it takes for a system to leave and return to the same thermodynamic state does not necessarily equal zero. However, if heat is supplied to a system in which no irreversible processes take place and which has a well-defined temperature T, the heat δQ and the temperature T form the exact differential
with S the entropy of the system. Likewise, with a well-defined pressure p behind the moving boundary, the work δW and the pressure p form the exact differential
with V the volume of the system. In general, for homogeneous systems,
Associated with this differential equation is that the internal energy may be considered to be a function U (S,V) of its natural variables S and V. Thus it is said that the internal energy representation of the fundamental thermodynamic relation is written
If V is constant
and if p is constant
with H the enthalpy given by
The enthalpy may be considered to be a function H (S,p) of its natural variables S and p. Thus it is said that the enthalpy representation of the fundamental thermodynamic relation is written
The internal energy representation and the enthalpy representation are partial Legendre transforms of one another. They contain the same physical information, written in different ways.[30][31]
Path-independent examples for an ideal gas
For a simple compressible system such as an ideal gas inside a piston, the internal energy change ΔU at constant volume and the enthalpy change ΔH at constant pressure are modeled by separate heat capacity values, which are CV and Cp, respectively.
Constrained to have constant volume, the heat, Q, required to change its temperature from an initial temperature, T0, to a final temperature, Tf, is given by
Allowing the system to expand or contract at constant pressure, the heat, Q, required to change its temperature from an initial temperature, T0, to a final temperature, Tf, is given by
Here we used the definition of the enthalpy and the fact that p is constant. When integrating an exact differential (e.g. dU), the lowercase letter d is substituted for Δ (e.g. ΔU). Note that the symbol Δ is convenient since it is compact, but it can lead to sign errors. So it may be better to write Uf - U0 instead of ΔU.
When integrating an inexact differential (e.g. δQ), the lowercase Greek letter δ is removed with no replacement (e.g. Q).
Chemical reactions
For a closed system in which a chemical reaction is of interest, the extent of reaction, denoted by ξ, states the degree of advancement of the reaction and is included as a further natural variable for internal energy and for enthalpy. This is written
In practice, chemists often use tables of a special but unnamed thermodynamic potential that is not the enthalpy expressed in its natural variables; instead they use the enthalpy expressed as a function of temperature instead of entropy. This special potential is related to the natural form of the enthalpy H (S,p,ξ) by another partial Legendre transform, that makes its natural variables T, p, and ξ. The special unnamed potential is still usually called the enthalpy. It can be written
This enthalpy is used to report the enthalpy change of reaction, also called the heat of reaction.[32][33]
Latent and sensible heat

In an 1847 lecture entitled On Matter, Living Force, and Heat, James Prescott Joule characterized the terms latent heat and sensible heat as components of heat each affecting distinct physical phenomena, namely the potential and kinetic energy of particles, respectively.[34] He described latent energy as the energy possessed via a distancing of particles where attraction was over a greater distance, i.e. a form of potential energy, and the sensible heat as an energy involving the motion of particles or what was known as a living force. At the time of Joule kinetic energy either held 'invisibly' internally or held 'visibly' externally was known as a living force.
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a change of state that occurs without a change in temperature. Such a process may be a phase transition, such as the melting of ice or the boiling of water.[35][36] The term was introduced around 1750 by Joseph Black as derived from the Latin latere (to lie hidden), characterizing its effect as not being directly measurable with a thermometer.
Sensible heat, in contrast to latent heat, is the heat exchanged by a thermodynamic system that has as its sole effect a change of temperature.[37] Sensible heat therefore only increases the thermal energy of a system.
Consequences of Black's distinction between sensible and latent heat are examined in the Wikipedia article on calorimetry.
Specific heat
Specific heat, also called specific heat capacity, is defined as the amount of energy that has to be transferred to or from one unit of mass (kilogram) or amount of substance (mole) to change the system temperature by one degree. Specific heat is a physical property, which means that it depends on the substance under consideration and its state as specified by its properties.
The specific heats of monatomic gases (e.g., helium) are nearly constant with temperature. Diatomic gases such as hydrogen display some temperature dependence, and triatomic gases (e.g., carbon dioxide) still more.
Entropy

In 1856, German physicist Rudolf Clausius defined the second fundamental theorem (the second law of thermodynamics) in the mechanical theory of heat (thermodynamics): "if two transformations which, without necessitating any other permanent change, can mutually replace one another, be called equivalent, then the generations of the quantity of heat Q from work at the temperature T, has the equivalence-value:"[38][39]
In 1865, he came to define the entropy symbolized by S, such that, due to the supply of the amount of heat Q at temperature T the entropy of the system is increased by
and thus, for small changes, quantities of heat δQ (an inexact differential) are defined as quantities of TdS, with dS an exact differential:
This equality is only valid for a closed system and if no irreversible processes take place inside the system while the heat δQ is applied. If, in contrast, irreversible processes are involved, e.g. some sort of friction, then there is entropy production and, instead of the above equation, one has
This is the second law of thermodynamics for closed systems.
Heat transfer in engineering

The discipline of heat transfer, typically considered an aspect of mechanical engineering and chemical engineering, deals with specific applied methods by which thermal energy in a system is generated, or converted, or transferred to another system. Although the definition of heat implicitly means the transfer of energy, the term heat transfer encompasses this traditional usage in many engineering disciplines and laymen language.
Heat transfer includes the mechanisms of heat conduction, thermal radiation, and mass transfer.
In engineering, the term convective heat transfer is used to describe the combined effects of conduction and fluid flow. From the thermodynamic point of view, heat flows into a fluid by diffusion to increase its energy, the fluid then transfers (advects) this increased internal energy (not heat) from one location to another, and this is then followed by a second thermal interaction which transfers heat to a second body or system, again by diffusion. This entire process is often regarded as an additional mechanism of heat transfer, although technically, "heat transfer" and thus heating and cooling occurs only on either end of such a conductive flow, but not as a result of flow. Thus, conduction can be said to "transfer" heat only as a net result of the process, but may not do so at every time within the complicated convective process.
Although distinct physical laws may describe the behavior of each of these methods, real systems often exhibit a complicated combination which are often described by a variety of complex mathematical methods.
Practical applications
In accordance with the first law for closed systems, energy transferred as heat enters one body and leaves another, changing the internal energies of each. Transfer, between bodies, of energy as work is a complementary way of changing internal energies. Though it is not logically rigorous from the viewpoint of strict physical concepts, a common form of words that expresses this is to say that heat and work are interconvertible.
Heat engines operate by converting heat flow from a high temperature reservoir to a low temperature reservoir into work. One example are steam engines, where the high temperature reservoir is steam generated by boiling water. The flow of heat from the hot steam to water is converted into mechanical work via a turbine or piston. Heat engines achieve high efficiency when the difference between initial and final temperature is high.
Heat pumps, by contrast, use work to cause thermal energy to flow from low to high temperature, the opposite direction heat would flow spontaneously. An example is a refrigerator or air conditioner, where electric power is used to cool a low temperature system (the interior of the refrigerator) while heating a higher temperature environment (the exterior). High efficiency is achieved when the temperature difference is small.
Usage of words
The strictly defined physical term 'quantity of energy transferred as heat' has a resonance with the ordinary language noun 'heat' and the ordinary language verb 'heat'. This can lead to confusion if ordinary language is muddled with strictly defined physical language. In the strict terminology of physics, heat is defined as a word that refers to a process, not to a state of a system. In ordinary language one can speak of a process that increases the temperature of a body as 'heating' it, ignoring the nature of the process, which could be one of adiabatic transfer of energy as work. But in strict physical terms, a process is admitted as heating only when what is meant is transfer of energy as heat. Such a process does not necessarily increase the temperature of the heated body, which may instead change its phase, for example by melting. In the strict physical sense, heat cannot be 'produced', because the usage 'production of heat' misleadingly seems to refer to a state variable. Thus, it would be physically improper to speak of 'heat production by friction', or of 'heating by adiabatic compression on descent of an air parcel' or of 'heat production by chemical reaction'; instead, proper physical usage speaks of conversion of kinetic energy of bulk flow, or of potential energy of bulk matter,[40] or of chemical potential energy, into internal energy, and of transfer of energy as heat. Occasionally a present-day author, especially when referring to history, writes of "adiabatic heating", though this is a contradiction in terms of present day physics.[41] Historically, before the concept of internal energy became clear over the period 1850 to 1869, physicists spoke of "heat production" where nowadays one speaks of conversion of other forms of energy into internal energy.[42]
See also
Notes
- ^ An alternate convention is to consider the work performed on the system by its surroundings. This leads to a change in sign of the work. This is the convention adopted by many modern textbooks of physical chemistry, such as those by Peter Atkins and Ira Levine, but many textbooks on physics define work as work done by the system.
References
- ^ Reif, F. (1965), pp. 67, 73.
- ^ a b Kittel, C. Kroemer, H. (1980). Thermal Physics, second edition, W.H. Freeman, San Francisco, ISBN 0-7167-1088-9, p. 227.
- ^ Guggenheim, E.A. (1949/1967), p. 8.
- ^ Planck. M. (1914). The Theory of Heat Radiation, a translation by Masius, M. of the second German edition, P. Blakiston's Son & Co., Philadelphia.
- ^ Maxwell 1871, Chapter III.
- ^ Kondepudi, D. (2008), p. 59.
- ^ Planck, M. (1927), p. 4.
- ^ Crawford, F.H. (1963). Heat, Thermodynamics, and Statistical Physics, Rupert Hart-Davis, Harcourt, Brace & World, p. 98.
- ^ Reif, F. (1965), p. 73.
- ^ Gislason, E.A., Craig, N.C. (2005). Cementing the foundations of thermodynamics: Comparison of system-based and surroundings-based definitions of heat and work, J. Chem. Thermodynamics, 37: 954–966.
- ^ Anacleto, J., Ferreira, J.M. (2008). Surroundings-based and system-based heat and work definitions: Which one is most suitable?, J. Chem. Thermodynamics, 40: 134–135.
- ^ Smith, J.M., Van Ness, H.C., Abbot, M.M. (2005). Introduction to Chemical Engineering Thermodynamics. McGraw-Hill. ISBN 0073104450.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Tolman, R.C. (1938). The Principles of Statistical Mechanics, Oxford University Press, Oxford UK, pp. 88, 93.
- ^ Bacon, F. (1620). Novum Organum Scientiarum, translated by Devey, J., P.F. Collier & Son, New York, 1902.
- ^ Partington, J.R. (1949), page 131.
- ^ Partington, J.R. (1949), pages 132–136.
- ^ Kittel, C. (1953/1980). Introduction to Solid State Physics, (first edition 1953), fifth edition 1980, John Wiley & Sons, New York, ISBN 0-471-49024-5, Chapters 4, 5.
- ^ Partington, J.R. (1949), p. 118.
- ^ Maxwell, J.C. (1871), p. 10.
- ^ Planck, M. (1903).
- ^ Partington, J.R. (1949).
- ^ Truesdell, C. (1980), page 15.
- ^ Maxwell J.C. (1872), p. 54.
- ^ Planck (1927), Chapter 3.
- ^ Bryan, G.H. (1907), p. 47.
- ^ C. Carathéodory (1909). "Untersuchungen über die Grundlagen der Thermodynamik". Mathematische Annalen. 67: 355–386. doi:10.1007/BF01450409. A partly reliable translation is to be found at Kestin, J. (1976). The Second Law of Thermodynamics, Dowden, Hutchinson & Ross, Stroudsburg PA.
- ^ Callen, H.B. (1985), Section 1-8.
- ^ Callen, H.B., (1985), Section 2-3, pp. 40–42.
- ^ a b Adkins, C.J. (1983), p. 101.
- ^ a b Callen, H.B. (1985), p. 147.
- ^ Adkins, C.J. (1983), pp. 100–104.
- ^ Prigogine, I.; Defay, R. (1954). Chemical Thermodynamics. translated by D.H. Everett. Longmans, Green & Co., London, Section 2-6, pp. 29–31.
- ^ Callen, H.B., (1985), Section 5-3, pp. 146–148, Section 6-6, pp. 173–179.
- ^ J. P. Joule (1884), The Scientific Paper of James Prescott Joule, The Physical Society of London, p. 274,
Heat must therefore consist of either living force or of attraction through space. In the former case we can conceive the constituent particles of heated bodies to be, either in whole or in part, in a state of motion. In the latter we may suppose the particles to be removed by the process of heating, so as to exert attraction through greater space. I am inclined to believe that both of these hypotheses will be found to hold good,—that in some instances, particularly in the case of sensible heat, or such as is indicated by the thermometer, heat will be found to consist in the living force of the particles of the bodies in which it is induced; whilst in others, particularly in the case of latent heat, the phenomena are produced by the separation of particle from particle, so as to cause them to attract one another through a greater space.
, Lecture on Matter, Living Force, and Heat. May 5 and 12, 1847 - ^ Perrot, Pierre (1998). A to Z of Thermodynamics. Oxford University Press. ISBN 0-19-856552-6.
- ^ Clark, John, O.E. (2004). The Essential Dictionary of Science. Barnes & Noble Books. ISBN 0-7607-4616-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Ritter, Michael E. (2006). "The Physical Environment: an Introduction to Physical Geography".
- ^ Published in Poggendoff’s Annalen, Dec. 1854, vol. xciii. p. 481; translated in the Journal de Mathematiques, vol. xx. Paris, 1855, and in the Philosophical Magazine, August 1856, s. 4. vol. xii, p. 81
- ^ Clausius, R. (1865). The Mechanical Theory of Heat –with its Applications to the Steam Engine and to Physical Properties of Bodies. London: John van Voorst, 1 Paternoster Row. MDCCCLXVII.
- ^ Iribarne, J.V., Godson, W.L. (1973/1989). Atmospheric thermodynamics, second edition, reprinted 1989, Kluwer Academic Publishers, Dordrecht, ISBN 90-277-1296-4, p. 136.
- ^ Bailyn, M. (1994), Part D, pp. 50–56.
- ^ For example, Clausius, R. (1857). Über die Art der Bewegung, welche wir Wärme nennen, Annalen der Physik und Chemie, 100: 353–380. Translated as On the nature of the motion which we call heat, Phil. Mag. series 4, 14: 108–128.
Bibliography
- Adkins, C.J. (1968/1983). Equilibrium Thermodynamics, (1st edition 1968), third edition 1983, Cambridge University Press, Cambridge UK, ISBN 0-521-25445-0.
- Bailyn, M. (1994). A Survey of Thermodynamics, American Institute of Physics Press, New York, ISBN 0-88318-797-3.
- Beattie, J.A., Oppenheim, I. (1979). Principles of Thermodynamics, Elsevier, Amsterdam, ISBN 0–444–41806–7.
- Bryan, G.H. (1907). Thermodynamics. An Introductory Treatise dealing mainly with First Principles and their Direct Applications, B.G. Teubner, Leipzig.
- Callen, H.B. (1960/1985). Thermodynamics and an Introduction to Thermostatistics, (1st edition 1960) 2nd edition 1985, Wiley, New York, ISBN 0-471-86256-8.
- Guggenheim, E.A. (1967) [1949], Thermodynamics. An Advanced Treatment for Chemists and Physicists (fifth ed.), Amsterdam: North-Holland Publishing Company.
{{citation}}: Cite has empty unknown parameters:|other=and|month=(help) - Haase, R. (1971). Survey of Fundamental Laws, chapter 1 of Thermodynamics, pages 1–97 of volume 1, ed. W. Jost, of Physical Chemistry. An Advanced Treatise, ed. H. Eyring, D. Henderson, W. Jost, Academic Press, New York, lcn 73–117081.
- Kondepudi, D. (2008), Introduction to Modern Thermodynamics, Chichester UK: Wiley, ISBN 978–0–470–01598–8.
{{citation}}: Check|isbn=value: invalid character (help); Cite has empty unknown parameters:|other=and|month=(help) - Kondepudi, D., Prigogine, I. (1998). Modern Thermodynamics: From Heat Engines to Dissipative Structures, John Wiley & Sons, Chichester, ISBN 0–471–97393–9.
- Maxwell, J.C. (1871), Theory of Heat (first ed.), London: Longmans, Green and Co.
{{citation}}: Cite has empty unknown parameters:|other=and|month=(help) - Partington, J.R. (1949), An Advanced Treatise on Physical Chemistry., vol. volume 1, Fundamental Principles. The Properties of Gases, London: Longmans, Green and Co.
{{citation}}:|volume=has extra text (help); Cite has empty unknown parameters:|other=and|month=(help) - Planck, M. (1903) [1897], Treatise on Thermodynamics (first ed.), London: Longmans, Green and Co.
{{citation}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|other=ignored (|others=suggested) (help) - Planck, M. (1927) [1923], Treatise on Thermodynamics (third ed.), London: Longmans, Green and Co.
{{citation}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|other=ignored (|others=suggested) (help) - Reif, F. (1965). Fundamentals of Statistical and Thermal Physics. New York: McGraw-Hll, Inc.
- Truesdell, C. (1980). The Tragicomical History of Thermodynamics 1822-1854, Springer, New York, ISBN 0–387–90403–4.
External links
- Heat on In Our Time at the BBC
- Plasma heat at 2 gigakelvins - Article about extremely high temperature generated by scientists (Foxnews.com)
- Correlations for Convective Heat Transfer - ChE Online Resources
- An Introduction to the Quantitative Definition and Analysis of Heat written for High School Students




















