Trimethylamine N-oxide: Difference between revisions
No edit summary |
BlueonGray (talk | contribs) m →Health issues: Non-objective assessment that appeals to the authority of a non-objective scientist who works for a non-objective, pro-meat advocacy organization. Has no place in an encyclopedia entry. |
||
| Line 67: | Line 67: | ||
==Health issues== |
==Health issues== |
||
A recent study indicates that the concentration of TMAO in the blood increases after consuming foods containing [[carnitine]]. High concentrations of carnitine are found in red meat, some [[energy drink]]s, and some [[dietary supplement]]s. Some types of [[Gut flora|gut bacteria]] (e.g. species of ''[[Acinetobacter]]'') convert dietary carnitine to TMAO. TMAO alters cholesterol metabolism in the intestines, in the liver, and in artery wall. In the presence of TMAO, there is increased deposition of cholesterol in, and decreased removal of cholesterol from, peripheral cells such as those in the artery wall.<ref name=Hazen>{{cite web|last=Hazen|first=Stanley|title=New Research On Red Meat And Heart Disease|url=http://thedianerehmshow.org/shows/2013-04-09/new-research-red-meat-and-heart-disease/transcript|work=The Diane Rehm Show (Transcript)|publisher=WAMU 88.5 American University Radio|accessdate=10 April 2013}}</ref> [[Vegan]]s and [[vegetarian]]s appear to have fewer gut bacteria that convert carnitine to TMAO.<ref>{{Cite news | author = [[Gina Kolata|Kolata, Gina]] | url = http://www.nytimes.com/2013/04/08/health/study-points-to-new-culprit-in-heart-disease.html | title = Study Points to a New Culprit in Heart Disease | publisher = ''[[The New York Times]]'' | date = April 7, 2013 | accessdate = 2013-04-07}}</ref><ref>{{cite journal | doi = 10.1038/nm.3145 | title = Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis | year = 2013 | last1 = Koeth | first1 = Robert A | last2 = Wang | first2 = Zeneng | last3 = Levison | first3 = Bruce S | last4 = Buffa | first4 = Jennifer A | last5 = Org | first5 = Elin | last6 = Sheehy | first6 = Brendan T | last7 = Britt | first7 = Earl B | last8 = Fu | first8 = Xiaoming | last9 = Wu | first9 = Yuping | journal = Nature Medicine}}</ref> |
A recent study indicates that the concentration of TMAO in the blood increases after consuming foods containing [[carnitine]]. High concentrations of carnitine are found in red meat, some [[energy drink]]s, and some [[dietary supplement]]s. Some types of [[Gut flora|gut bacteria]] (e.g. species of ''[[Acinetobacter]]'') convert dietary carnitine to TMAO. TMAO alters cholesterol metabolism in the intestines, in the liver, and in artery wall. In the presence of TMAO, there is increased deposition of cholesterol in, and decreased removal of cholesterol from, peripheral cells such as those in the artery wall.<ref name=Hazen>{{cite web|last=Hazen|first=Stanley|title=New Research On Red Meat And Heart Disease|url=http://thedianerehmshow.org/shows/2013-04-09/new-research-red-meat-and-heart-disease/transcript|work=The Diane Rehm Show (Transcript)|publisher=WAMU 88.5 American University Radio|accessdate=10 April 2013}}</ref> [[Vegan]]s and [[vegetarian]]s appear to have fewer gut bacteria that convert carnitine to TMAO.<ref>{{Cite news | author = [[Gina Kolata|Kolata, Gina]] | url = http://www.nytimes.com/2013/04/08/health/study-points-to-new-culprit-in-heart-disease.html | title = Study Points to a New Culprit in Heart Disease | publisher = ''[[The New York Times]]'' | date = April 7, 2013 | accessdate = 2013-04-07}}</ref><ref>{{cite journal | doi = 10.1038/nm.3145 | title = Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis | year = 2013 | last1 = Koeth | first1 = Robert A | last2 = Wang | first2 = Zeneng | last3 = Levison | first3 = Bruce S | last4 = Buffa | first4 = Jennifer A | last5 = Org | first5 = Elin | last6 = Sheehy | first6 = Brendan T | last7 = Britt | first7 = Earl B | last8 = Fu | first8 = Xiaoming | last9 = Wu | first9 = Yuping | journal = Nature Medicine}}</ref> |
||
The vilification of red meat is puzzling, however, when the data from the study is viewed. For example, halibut generated "over 107 times as much TMAO as red meat." <ref>{{cite web|last=Masterjohn|first=Chris|title=Does Carnitine From Red Meat Contribute to Heart Disease Through Intestinal Bacterial Metabolism to TMAO?|url=http://www.westonaprice.org/blogs/cmasterjohn/2013/04/10/does-carnitine-from-red-meat-contribute-to-heart-disease-through-intestinal-bacterial-metabolism-to-tmao/|accessdate=11 April 2013}}</ref> |
|||
==References== |
==References== |
||
Revision as of 01:19, 12 April 2013
| |

| |
| Names | |
|---|---|
| IUPAC name
trimethylamine oxide
| |
| Preferred IUPAC name
N,N-dimethylmethanamine oxide | |
| Other names
trimethylamine oxide, TMAO, TMANO
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.341 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H9NO | |
| Molar mass | 75.11 |
| Appearance | colourless solid |
| Melting point | 220–222 °C (hydrate: 96 °C) |
| good | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
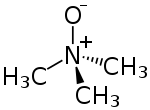
Trimethylamine N-oxide (TMAO) is the organic compound with the formula (CH3)3NO. This colorless solid is usually encountered as the dihydrate. It is an oxidation product of trimethylamine and a common metabolite in animals. It is an osmolyte found in saltwater fish, sharks and rays, molluscs, and crustaceans. It is a protein stabilizer that may serve to counteract urea, the major osmolyte of sharks, skates and rays. It is also higher in deep-sea fishes and crustaceans, where it may counteract the protein-destabilizing effects of pressure.[1] TMAO decomposes to trimethylamine (TMA), which is the main odorant that is characteristic of degrading seafood.
Synthesis
Treatment of aqueous trimethylamine with hydrogen peroxide affords the dihydrate (Me = CH3):[2]
- H2O2 + Me3N → H2O + Me3NO
Trimethylamine-N-oxide is biosynthesized from trimethylamine, which is derived from choline.[3]
Trimethylaminuria
Trimethylaminuria is a defect in the production of the enzyme flavin containing monooxygenase 3 (FMO3),[4][5] causing incomplete breakdown of trimethylamine from choline-containing food into trimethylamine oxide. Trimethylamine then builds up and is released in the person's sweat, urine, and breath, giving off a strong fishy odor.
Laboratory applications
Trimethylamine oxide is used in protein folding experiments to counteract the unfolding effects of urea.[6]
In the organometallic chemistry reaction of nucleophilic abstraction, Me3NO is employed as a decarbonylation agent according to the following stoichiometry:
- M(CO)n + Me3NO + L → M(CO)n-1L + Me3N + CO2
This reaction is used to decomplex organic ligands from metals, e.g. from (diene)Fe(CO)3.[2]
It is used in certain oxidation reactions, e.g. the conversion of alkyl iodides to the aldehyde.[7]
Health issues
A recent study indicates that the concentration of TMAO in the blood increases after consuming foods containing carnitine. High concentrations of carnitine are found in red meat, some energy drinks, and some dietary supplements. Some types of gut bacteria (e.g. species of Acinetobacter) convert dietary carnitine to TMAO. TMAO alters cholesterol metabolism in the intestines, in the liver, and in artery wall. In the presence of TMAO, there is increased deposition of cholesterol in, and decreased removal of cholesterol from, peripheral cells such as those in the artery wall.[8] Vegans and vegetarians appear to have fewer gut bacteria that convert carnitine to TMAO.[9][10]
References
- ^ Yancey, P. (2005). "Organic osmolytes as compatible, metabolic, and counteracting cytoprotectants in high osmolarity and other stresses". J. Exp. Biol. 208 (15): 2819–2830. doi:10.1242/jeb.01730. PMID 16043587.
- ^ a b A. J. Pearson "Trimethylamine N-Oxide" in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, 2001: New York. doi:10.1002/047084289X.rt268
- ^ Baker, J.R.; Chaykin, S. (1 April 1962). "The biosynthesis of trimethylamine-N-oxide". J. Biol. Chem. 237 (4): 1309–13. PMID 13864146.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Treacy, E.P.; Akerman, BR; et al. (1998). "Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication". Human Molecular Genetics. 7 (5): 839–45. doi:10.1093/hmg/7.5.839. PMID 9536088.
- ^ Zschocke J, Kohlmueller D, Quak E, Meissner T, Hoffmann GF, Mayatepek E (1999). "Mild trimethylaminuria caused by common variants in FMO3 gene". Lancet. 354 (9181): 834–5. doi:10.1016/S0140-6736(99)80019-1. PMID 10485731.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zou, Q.; et al. (2002). "The Molecular Mechanism of Stabilization of Proteins by TMAO and Its Ability to Counteract the Effects of Urea". J. Am. Chem. Soc. 124 (7): 1192–1202. doi:10.1021/ja004206b. PMID 11841287.
- ^ Volker Franzen (1973). "Octanal". Organic Syntheses; Collected Volumes, vol. 5, p. 872.
- ^ Hazen, Stanley. "New Research On Red Meat And Heart Disease". The Diane Rehm Show (Transcript). WAMU 88.5 American University Radio. Retrieved 10 April 2013.
- ^ Kolata, Gina (April 7, 2013). "Study Points to a New Culprit in Heart Disease". The New York Times. Retrieved 2013-04-07.
{{cite news}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Koeth, Robert A; Wang, Zeneng; Levison, Bruce S; Buffa, Jennifer A; Org, Elin; Sheehy, Brendan T; Britt, Earl B; Fu, Xiaoming; Wu, Yuping (2013). "Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis". Nature Medicine. doi:10.1038/nm.3145.
