Germane: Difference between revisions
m →External links: added link with chemical safety info |
m →Safety |
||
| Line 80: | Line 80: | ||
==Safety== |
==Safety== |
||
Germane is a highly [[flammable]], potentially [[pyrophoric]],<ref>Brauer, 1963, Vol.1, 715</ref> and highly toxic gas. In 1970, the [[American Conference of Governmental Industrial Hygienists]] (ACGIH) published the latest changes and set the occupational exposure threshold limit value at 0.2 [[Parts per million|ppm]] for an 8 hour time weighted average.<ref name="Praxair MSDS">[http://www.praxair.com/praxair.nsf/AllContent/9ACB6769F6CDBEE785257258006F2F39/$File/p4821e.pdf Praxair MSDS] accessed Sep. 2011</ref> |
Germane is a highly [[flammable]], potentially [[pyrophoric]],<ref>Brauer, 1963, Vol.1, 715</ref> and highly toxic gas. In 1970, the [[American Conference of Governmental Industrial Hygienists]] (ACGIH) published the latest changes and set the occupational exposure threshold limit value at 0.2 [[Parts per million|ppm]] for an 8 hour time weighted average.<ref name="Praxair MSDS">[http://www.praxair.com/praxair.nsf/AllContent/9ACB6769F6CDBEE785257258006F2F39/$File/p4821e.pdf Praxair MSDS] accessed Sep. 2011</ref> |
||
The [[LC50]] for rats at 1 hour of exposure is 622 ppm.<ref>[http://www.cdc.gov/niosh-rtecs/LY4AC4A0.html#VCVN1 |
The [[LC50]] for rats at 1 hour of exposure is 622 ppm.<ref>[http://www.cdc.gov/niosh-rtecs/LY4AC4A0.html#VCVN1 NIOSH Germane] [[Registry of Toxic Effects of Chemical Substances]] (RTECS)accessed Sep. 2011</ref> Inhalation exposure may result in malaise, headache, dizziness, fainting, dyspnea, nausea, vomiting, kidney injury, and hemolytic effects.<ref>{{ cite journal | author = Gus'kova, E. I. | title = K toksikologii Gidrida Germaniia | trans_title = Toxicology of germanium hydride | language = Russian | journal = Gigiena Truda i Professionalnye Zabolevaniia | year = 1974 | volume = 18 | issue = 2 | pages = 56–57 | pmid = 4839911 }}</ref><ref>[http://www.epa.gov/oppt/aegl/pubs/germane_interim_sep_09_v1.pdf US EPA Germane]</ref><ref>{{ cite journal | author = Paneth, F.; Joachimoglu, G. | title = Über die pharmakologischen Eigenschaften des Zinnwasserstoffs und Germaniumwasserstoffs | trans_title = About the pharmacological characteristics of tin hydride and germanium hydride | language = German | journal = [[Berichte der Deutschen Chemischen Gesellschaft]] | year = 1924 | volume = 57 | issue = 10 | pages = 1925–1930 | doi = 10.1002/cber.19240571027 }}</ref> |
||
The [[US Department of Transportation]] [[Dangerous goods|hazard class]] is 2.3 Poisonous Gas.<ref name="Praxair MSDS"/> |
The [[US Department of Transportation]] [[Dangerous goods|hazard class]] is 2.3 Poisonous Gas.<ref name="Praxair MSDS"/> |
||
Revision as of 16:56, 19 November 2013
- For other meanings see germane.
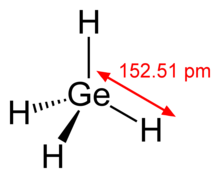
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Germane
| |||
| Other names
Germanium tetrahydride
Germanomethane Monogermane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.055 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UN number | 2192 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| GeH4 | |||
| Molar mass | 76.62 g/mol1 | ||
| Appearance | Colorless gas | ||
| Density | 3.3 kg/m3 gas | ||
| Melting point | −165 °C (108 K) | ||
| Boiling point | −88 °C (185 K) | ||
| low | |||
| Structure | |||
| Tetrahedral | |||
| O D | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic, flammable | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water.
Occurrence
Germane has been detected in the atmosphere of Jupiter.[1]
Synthesis
Germane is typically prepared by reduction of germanium compounds with hydride reagents such as sodium borohydride, potassium borohydride, lithium borohydride, lithium aluminium hydride, sodium aluminium hydride. The reaction is catalyzed by various acids and can be carried out in either aqueous or in an organic solvent. On laboratory scale, germane can be prepared by the reaction of Ge(IV) compounds with these hydride reagents.[2][3] A typical synthesis involved the reaction of Na2GeO3 with sodium borohydride.[4]
- Na2GeO3 + NaBH4 + H2O → GeH4 + 2 NaOH + NaBO2
Other methods for the synthesis of germane include electrochemical reduction and a plasma-based method.[5] The electrochemical reduction method involves applying voltage to a germanium metal cathode immersed in an aqueous electrolyte solution and an anode counter-electrode composed of a metal such as molybdenum or cadmium. In this method, germane and hydrogen gases evolve from the cathode while the anode reacts to form solid molybdenum oxide or cadmium oxides. The plasma synthesis method involves bombarding germanium metal with hydrogen atoms (H) that are generated using a high frequency plasma source to produce germane and digermane.
Reactions
In liquid ammonia GeH4 is ionised forming NH4+ and GeH3−.[6] With alkali metals in liquid ammonia GeH4 reacts to give white crystalline MGeH3 compounds. The potassium and rubidium compounds have the sodium chloride structure implying a free rotation of the GeH3− anion, the caesium compound, CsGeH3 in contrast has the distorted sodium chloride structure of TlI.[6]
Use in semiconductor industry
The gas decomposes near 600K to germanium and hydrogen. Because of its thermal lability, germane is used in the semiconductor industry for the epitaxial growth of germanium by MOVPE or chemical beam epitaxy.[7] Organogermanium precursors (e.g. isobutylgermane, alkylgermanium trichlorides, and dimethylaminogermanium trichloride) have been examined as less hazardous liquid alternatives to germane for deposition of Ge-containing films by MOVPE.[8]
Safety
Germane is a highly flammable, potentially pyrophoric,[9] and highly toxic gas. In 1970, the American Conference of Governmental Industrial Hygienists (ACGIH) published the latest changes and set the occupational exposure threshold limit value at 0.2 ppm for an 8 hour time weighted average.[10] The LC50 for rats at 1 hour of exposure is 622 ppm.[11] Inhalation exposure may result in malaise, headache, dizziness, fainting, dyspnea, nausea, vomiting, kidney injury, and hemolytic effects.[12][13][14]
The US Department of Transportation hazard class is 2.3 Poisonous Gas.[10]
References
- ^ Kunde, V.; Hanel, R.; Maguire, W.; Gautier, D.; Baluteau, J. P.; Marten, A.; Chedin, A.; Husson, N.; Scott, N. (1982). "The tropospheric gas composition of Jupiter's north equatorial belt (NH3, PH3, CH3D, GeH4, H2O) and the Jovian D/H isotopic ratio". Astrophysical Journal. 263: 443–467. Bibcode:1982ApJ...263..443K. doi:10.1086/160516.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ W. L. Jolly "Preparation of the volatile hydrides of Groups IVA and VA by means of aqueous hydroborate" Journal of the American Chemical Society 1961, volume 83, pp. 335-7.
- ^ US Patent 4,668,502
- ^ Girolami, G. S.; Rauchfuss, T. B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry. Mill Valley, CA: University Science Books.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ US Patent 7,087,102 (2006)
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Venkatasubramanian, R.; Pickett, R. T.; Timmons, M. L. (1989). "Epitaxy of germanium using germane in the presence of tetramethylgermanium". Journal of Applied Physics. 66 (11): 5662–5664. Bibcode:1989JAP....66.5662V. doi:10.1063/1.343633.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Woelk, E.; Shenai-Khatkhate, D. V.; DiCarlo, R. L. Jr., Amamchyan, A.; Power, M. B.; Lamare, B.; Beaudoin, G.; Sagnes, I. (2006). "Designing Novel Organogermanium MOVPE Precursors for High-purity Germanium Films". Journal of Crystal Growth. 287 (2): 684–687. Bibcode:2006JCrGr.287..684W. doi:10.1016/j.jcrysgro.2005.10.094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brauer, 1963, Vol.1, 715
- ^ a b Praxair MSDS accessed Sep. 2011
- ^ NIOSH Germane Registry of Toxic Effects of Chemical Substances (RTECS)accessed Sep. 2011
- ^ Gus'kova, E. I. (1974). "K toksikologii Gidrida Germaniia". Gigiena Truda i Professionalnye Zabolevaniia (in Russian). 18 (2): 56–57. PMID 4839911.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - ^ US EPA Germane
- ^ Paneth, F.; Joachimoglu, G. (1924). "Über die pharmakologischen Eigenschaften des Zinnwasserstoffs und Germaniumwasserstoffs". Berichte der Deutschen Chemischen Gesellschaft (in German). 57 (10): 1925–1930. doi:10.1002/cber.19240571027.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)CS1 maint: multiple names: authors list (link)



