Photoelectrochemical cell: Difference between revisions
grammar |
clarifying difference with standard photovoltaic cell |
||
| Line 1: | Line 1: | ||
{{Use American English|date = April 2019}} |
{{Use American English|date = April 2019}} |
||
{{Short description|sources of electricity or hydrogen via electrolysis}} |
{{Short description|sources of electricity or hydrogen via electrolysis}} |
||
"'''Photoelectrochemical cell'''" refers to one of two different devices. The first [[electricity generation|produces electrical energy]] similarly to a [[dye-sensitized solar cell]]. The second is a '''photoelectrolytic cell''', that is, a device which uses light to directly cause a chemical reaction, for example to produce [[hydrogen]] via the [[electrolysis of water]]. |
"'''Photoelectrochemical cell'''" refers to one of two different devices. The first [[electricity generation|produces electrical energy]] similarly to a [[dye-sensitized solar cell|dye-sensitized photovoltaic cell]]. The second is a '''photoelectrolytic cell''', that is, a device which uses light to directly cause a chemical reaction, for example to produce [[hydrogen]] via the [[electrolysis of water]]. |
||
Both types of device are varieties of [[solar cells|solar cell]], in that a photoelectrochemical cell's function is to use the [[photoelectric effect]] (or, very similarly, the [[photovoltaic effect]]) to convert [[electromagnetic radiation]] (typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to [[Hydrogen fuel|create electrical power]], see [[photohydrogen]]). |
Both types of device are varieties of [[solar cells|solar cell]], in that a photoelectrochemical cell's function is to use the [[photoelectric effect]] (or, very similarly, the [[photovoltaic effect]]) to convert [[electromagnetic radiation]] (typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to [[Hydrogen fuel|create electrical power]], see [[photohydrogen]]). |
||
The standard [[photovoltaic effect]], as operating in standard [[solar cells|photovoltaic cells]], however, involves the excitation of negative charge carriers (electrons) within a semiconductor medium, and it is negative charge carriers (free electrons) which are ultimately are extracted to produce power. On the other hand, within a water-splitting photoelectrolytic cell, for example, the excitation, by light, of an electron in a semiconductor leaves a hole which "draws" an electron from a neighboring water molecule: |
|||
:::::::::::::<chem>H2O(l) + [hv] + h+ -> H+ (aq) + O2(g)</chem> |
|||
This leaves positive charge carriers (protons, that is, H+ ions) in solution, which must then bond with one other proton and combine with two electrons in order to form hydrogen gas, according to: |
|||
:::::::::::::<chem>2H+ + 2e- -> H2(g)</chem> |
|||
Revision as of 08:27, 29 June 2019
"Photoelectrochemical cell" refers to one of two different devices. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.
Both types of device are varieties of solar cell, in that a photoelectrochemical cell's function is to use the photoelectric effect (or, very similarly, the photovoltaic effect) to convert electromagnetic radiation (typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to create electrical power, see photohydrogen).
The standard photovoltaic effect, as operating in standard photovoltaic cells, however, involves the excitation of negative charge carriers (electrons) within a semiconductor medium, and it is negative charge carriers (free electrons) which are ultimately are extracted to produce power. On the other hand, within a water-splitting photoelectrolytic cell, for example, the excitation, by light, of an electron in a semiconductor leaves a hole which "draws" an electron from a neighboring water molecule:
This leaves positive charge carriers (protons, that is, H+ ions) in solution, which must then bond with one other proton and combine with two electrons in order to form hydrogen gas, according to:
Photoelectrolytic cell
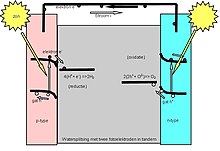
A photoelectrolytic cell, sometimes called a photogeneration cell[1] electrolizes water into hydrogen and oxygen gas by irradiating the anode with electromagnetic radiation, that is, with light. This has been referred to as artificial photosynthesis and has been suggested as a way of storing solar energy in hydrogen for use as fuel.[2]
Incoming sunlight excites free electrons near the surface of the silicon electrode. These electrons flow through wires to the stainless steel electrode, where four of them react with four water molecules to form two molecules of hydrogen and 4 OH groups. The OH groups flow through the liquid electrolyte to the surface of the silicon electrode. There they react with the four holes associated with the four photoelectrons, the result being two water molecules and an oxygen molecule. Illuminated silicon immediately begins to corrode under contact with the electrolytes. The corrosion consumes material and disrupts the properties of the surfaces and interfaces within the cell.[3]
Two types of photochemical systems operate via photocatalysis. One uses semiconductor surfaces as catalysts. In these devices the semiconductor surface absorbs solar energy and acts as an electrode for water splitting. The other methodology uses in-solution metal complexes as catalysts.[4][5]
Photoelectrolytic cells have passed the 10 percent economic efficiency barrier. Corrosion of the semiconductors remains an issue, given their direct contact with water.[6] Research is now ongoing to reach a service life of 10000 hours, a requirement established by the United States Department of Energy.[7]
Other Photoelectrochemical Cells
The first photovoltaic cell ever designed was also the first photoelectrochemical cell. It was created in 1839, by Alexandre-Edmond Becquerel, at age 19, in his father's laboratory.[8]
The mostly commonly researched modern photoelectrochemical cell in recent decades has been the Grätzel cell, although much attention has recently shifted away from this topic to perovskite solar cells, due to relatively high efficiency of the latter and the similarity in vapor assisted deposition techniques commonly used in their creation.
Dye-sensitized solar cells or Grätzel cells use dye-adsorbed highly porous nanocrystalline titanium dioxide (nc-TiO
2) to produce electrical energy.
Materials for Photoelectrolytic Cells
Photoelectrolytic Photoelectrochemical cells (PECs) convert light energy into electricity within a two-electrode cell. In theory, three arrangements of photo-electrodes in the assembly of PECs exist:[9]
- photo-anode made of a n-type semiconductor and a metal cathode
- photo-anode made of a n-type semiconductor and a photo-cathode made of a p-type semiconductor
- photo-cathode made of a p-type semiconductor and a metal anode
The two basic requirements for materials used as photo-electrodes are optical function, required to obtain maximal absorption of solar energy, and catalytic function, required for other reactions such as water decomposition.
TiO
2
In 1967, Akira Fujishima discovered the Honda-Fujishima effect, (the photocatalytic properties of titanium dioxide).
TiO
2 and other metal oxides are still most prominent[10] catalysts for efficiency reasons. Including SrTiO
3 and BaTiO
3,[11] this kind of semiconducting titanates, the conduction band has mainly titanium 3d character and the valence band oxygen 2p character. The bands are separated by a wide band gap of at least 3 eV, so that these materials absorb only UV radiation. Change of the TiO
2 microstructure has also been investigated to further improve the performance, such as TiO
2 nanowire arrays or porous nanocrystalline TiO
2 photoelectrochemical cells.[12]
GaN
GaN is another option, because metal nitrides usually have a narrow band gap that could encompass almost the entire solar spectrum.[13] GaN has a narrower band gap than TiO
2 but is still large enough to allow water splitting to occur at the surface. GaN nanowires exhibited better performance than GaN thin films, because they have a larger surface area and have a high single crystallinity which allows longer electron-hole pair lifetimes.[14] Meanwhile, other non-oxide semiconductors such as GaAs, MoS
2, WSe
2 and MoSe
2 are used as n-type electrode, due to their stability in chemical and electrochemical steps in the photocorrosion reactions.[15]
Silicon
In 2013 a cell with 2 nanometers of nickel on a silicon electrode, paired with a stainless steel electrode, immersed in an aqueous electrolyte of potassium borate and lithium borate operated for 80 hours without noticeable corrosion, versus 8 hours for titanium dioxide. In the process, about 150 ml of hydrogen gas was generated, representing the storage of about 2 kilojoules of energy.[3][16]
See also
References
- ^ https://www.revolvy.com/page/Photoelectrochemical-cell
- ^ John A. Turner; et al. (2007-05-17). "Photoelectrochemical Water Systems for H2 Production" (PDF). National Renewable Energy Laboratory. Archived from the original (PDF) on 2011-06-11. Retrieved 2011-05-02.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ^ a b "Silicon/nickel water splitter could lead to cheaper hydrogen". Gizmag.com. Retrieved 2013-12-29.
- ^ Berinstein, Paula (2001-06-30). Alternative energy: facts, statistics, and issues. Greenwood Publishing Group. ISBN 1-57356-248-3.
Another photoelectrochemical method involves using dissolved metal complexes as a catalyst, which absorbs energy and creates an electric charge separation that drives the water-splitting reaction.
- ^ Deutsch, T. G.; Head, J. L.; Turner, J. A. (2008). "Photoelectrochemical Characterization and Durability Analysis of GaInPN Epilayers". Journal of the Electrochemical Society. 155 (9): B903. doi:10.1149/1.2946478.
- ^ Brad Plummer (2006-08-10). "A Microscopic Solution to an Enormous Problem". SLAC Today. SLAC National Accelerator Laboratory. Retrieved 2011-05-02.
- ^ Wang, H.; Deutsch, T.; Turner, J. A. A. (2008). "Direct Water Splitting Under Visible Light with a Nanostructured Photoanode and GaInP2 Photocathode". ECS Transactions. 6: 37. doi:10.1149/1.2832397.
- ^ https://www.pveducation.org/pvcdrom/manufacturing/first-photovoltaic-devices
- ^ Tryk, D.; Fujishima, A; Honda, K (2000). "Recent topics in photoelectrochemistry: achievements and future prospects". Electrochimica Acta. 45 (15–16): 2363–2376. doi:10.1016/S0013-4686(00)00337-6.
- ^ A. Fujishima, K. Honda, S. Kikuchi, Kogyo Kagaku Zasshi 72 (1969) 108–113
- ^ De Haart, L.; De Vries, A. J.; Blasse, G. (1985). "On the photoluminescence of semiconducting titanates applied in photoelectrochemical cells". Journal of Solid State Chemistry. 59 (3): 291–300. Bibcode:1985JSSCh..59..291D. doi:10.1016/0022-4596(85)90296-8.
- ^ Cao, F.; Oskam, G.; Meyer, G. J.; Searson, P. C. (1996). "Electron Transport in Porous Nanocrystalline TiO2Photoelectrochemical Cells". The Journal of Physical Chemistry. 100 (42): 17021–17027. doi:10.1021/jp9616573.
- ^ Wang, D.; Pierre, A.; Kibria, M. G.; Cui, K.; Han, X.; Bevan, K. H.; Guo, H.; Paradis, S.; Hakima, A. R.; Mi, Z. (2011). "Wafer-Level Photocatalytic Water Splitting on GaN Nanowire Arrays Grown by Molecular Beam Epitaxy". Nano Letters. 11 (6): 2353–2357. Bibcode:2011NanoL..11.2353W. doi:10.1021/nl2006802. PMID 21568321.
- ^ Jung, Hye Song; Young Joon Hong, Yirui Li, Jeonghui Cho, Young-Jin Kim, Gyu-Chui Yi (2008). "Photocatalysis Using GaN Nanowires". ACS Nano. 2 (4): 637–642. doi:10.1021/nn700320y.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kline, G.; Kam, K.; Canfield, D.; Parkinson, B. (1981). "Efficient and stable photoelectrochemical cells constructed with WSe2 and MoSe2 photoanodes". Solar Energy Materials. 4 (3): 301–308. doi:10.1016/0165-1633(81)90068-X.
- ^ Kenney, M. J.; Gong, M.; Li, Y.; Wu, J. Z.; Feng, J.; Lanza, M.; Dai, H. (2013). "High-Performance Silicon Photoanodes Passivated with Ultrathin Nickel Films for Water Oxidation". Science. 342 (6160): 836–840. Bibcode:2013Sci...342..836K. doi:10.1126/science.1241327. PMID 24233719.

![{\displaystyle {\ce {H2O(l) + [hv] + h+ -> H+ (aq) + O2(g)}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/20a478f92dc37c212bf6c394f80703e9c5d80586)
