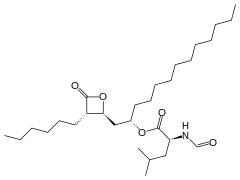
Orlistat
 | |
| File:Orlistat 3D sticks.png | |
| Clinical data | |
|---|---|
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Negligible |
| Protein binding | >99% |
| Metabolism | In the GI tract |
| Elimination half-life | 1 to 2 hours |
| Excretion | Fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.400 |
| Chemical and physical data | |
| Formula | C29H53NO5 |
| Molar mass | 495.735 g/mol g·mol−1 |
Orlistat (marketed under the trade name Xenical by Roche or over-the-counter as alli by GlaxoSmithKline) — also known as tetrahydrolipstatin — is a drug designed to treat obesity.[1] Its primary function is preventing the absorption of fats from the human diet, thereby reducing caloric intake. It is intended for use in conjunction with a physician-supervised reduced calorie diet.
Orlistat is the saturated derivative of lipstatin — a potent natural inhibitor of pancreatic lipases isolated from the bacterium Streptomyces toxytricini.[2] However, due to simplicity and stability, orlistat rather than lipstatin was developed into an anti-obesity drug.[3]
Pharmacology
Orlistat works by inhibiting pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. Without this enzyme, triglycerides from the diet are prevented from being hydrolyzed into absorbable free fatty acids and are excreted undigested. Only trace amounts of orlistat are absorbed systemically; the primary effect is local lipase inhibition within the GI tract after an oral dose. The primary route of elimination is through the feces.
At the standard prescription dose of 120 mg three times daily before meals, orlistat prevents approximately 30% of dietary fat from being absorbed.[4] Higher doses do not produce more potent effects.[5]
Efficacy
The amount of weight loss achieved with orlistat varies. In one-year clinical trials, between 35.5% and 54.8% of subjects achieved a 5% or greater decrease in body mass, although not all of this mass was necessarily fat. Between 16.4% and 24.8% achieved at least a 10% decrease in body mass.[5] After orlistat was stopped, a significant number of subjects regained weight — up to 35% of the weight they had lost.[5] Despite this relatively small body mass effect, there was a 37% reduction in the incidence of Type 2 diabetes,[6] a significant difference.
Side effects
The primary side effects of the drug are gastrointestinal-related. Side effects are most severe when beginning therapy, and decrease in frequency with time; in clinical trials, nearly half of side effects lasted less than a week, but some may persist for over six months.[7] Because orlistat's main effect is to prevent dietary fat from being absorbed, the fat is excreted unchanged in the feces and so the stool may become oily or loose (steatorrhea). Increased flatulence is also common. Bowel movements may become frequent or urgent, and rare occurrences of fecal incontinence have been seen in clinical trials. To minimize these effects, foods with high fat content should be avoided; the manufacturer advises consumers to follow a low-fat, reduced-calorie diet. Oily stools and flatulence can be controlled by reducing the dietary fat content to somewhere in the region of 15 grams per meal.[8]
Absorption of fat-soluble vitamins and other fat-soluble nutrients is inhibited by the use of orlistat. A multivitamin tablet containing vitamins A, D, E, K, and beta-carotene should be taken once a day, at least 2 hours before or after taking the drug.[7]
Despite claims that orlistat increases the risk of breast cancer amongst clinical trial participants, there is evidence to suggest that the introduction of specific varied preparations containing orlistat, namely the concurrent administration of orlistat and the monoclonal antibody trastuzumab, can actually induce cell death in tumor cells and block their growth.[9]
A 2006 animal study addressed a connection orlistat shares with aberrant crypt foci (ACF), lesions found in the colon which are believed to be one of the earliest precursors of colon cancer.[10][11]
Interactions
Orlistat may reduce plasma levels of ciclosporin, an immunosuppressive drug frequently used to prevent transplant rejection; the two drugs should therefore not be administered concomitantly.[7]
Contraindications
Orlistat is contraindicated in:[7]
- Malabsorption
- Reduced gallbladder function (e.g. after cholecystectomy)
- Pregnancy and breastfeeding
Availability
In most areas, such as the United Kingdom, France, and Canada, orlistat is available by prescription only. In Australia and the United States, certain formulations of orlistat have been approved for sale without a prescription.
Australia
In Australia, orlistat is currently available over-the-counter in 120 mg size (84 capsules to the pack). Initially available only with a prescription, it was reclassified as a "Pharmacist Only Medicine" in October 2003. In late 2006, the Australian Consumers' Association complained that Roche was inappropriately advertising the drug to teenagers, and Roche was forced to withdraw its ads.[12] The Association filed further complaints[12] with the Therapeutic Goods Administration — TGA, Australia's regulatory authority for healthcare products — and the TGA's Scheduling Committee agreed to convene on February 20, 2007, to discuss possible revoking of orlistat's over-the-counter status.[13] The Committee ultimately decided to keep orlistat as a Schedule 3 drug, but withdrew its authorization of direct-to-consumer Xenical advertising, stating this "increased pressure on pharmacists to provide orlistat to consumers...this in turn had the potential to result in inappropriate patterns of use".[14]
United States
On January 23, 2006, a U.S. Food and Drug Administration advisory panel voted 11 to 3 to recommend the approval of an OTC formulation of orlistat, to be marketed under the name alli by GlaxoSmithKline.[15] Approval was granted on February 7, 2007,[16] and alli became the first weight loss drug officially sanctioned by the U.S. government for over-the-counter use.[17] Consumer advocacy organization Public Citizen, through its Health Research Group, opposed over-the-counter approval for orlistat, calling it "the height of recklessness" and "a dangerous mistake" due to questionable benefits and possible adverse effects.[18]
Becoming available in the U.S. during the summer of 2007, alli will be sold as 60 mg capsules — half the dosage of prescription orlistat.[17][18]
Generic formulations
As of 2007, no generic formulations of orlistat are legally available in the United States. U.S. patent protection for Xenical, originally to end on June 18, 2004, was extended by five years (until 2009) by the U.S. Patent and Trademark Office. The extension was granted on July 20, 2002.[19]
References
- ^ Bodkin J, Humphries E, McLeod M (2003). "The total synthesis of (−)-tetrahydrolipstatin". Australian Journal of Chemistry. 56 (8): 795–803. doi:10.1071/CH03121.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barbier P, Schneider F (1987). "Syntheses of tetrahydrolipstatin and absolute configuration of tetrahydrolipstatin and lipstatin". Helvetica Chimica Acta. 70 (1): 196–202. doi:10.1002/hlca.19870700124.
- ^ Pommier A, Pons M, Kocienski P (1995). "The first total synthesis of (-)-lipstatin". Journal of Organic Chemistry. 60 (22): 7334–7339. doi:10.1021/jo00127a045.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ 2006 Physicians' Desk Reference (PDR). Thomson PDR. 2006. ISBN 1-56363-527-5.
- ^ a b c "Xenical Pharmacology, Pharmacokinetics, Studies, Metabolism". RxList.com. 2007. Retrieved 2007-03-16.
- ^ Torgerson J, Hauptman J, Boldrin M, Sjöström L (2004). "XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients". Diabetes Care. 27 (1): 155–61. PMID 14693982.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Roche Pharmaceuticals (January 5, 2007). "Xenical® Product Label" (PDF, 300 KiB). U.S. Food and Drug Administration. Retrieved 2007-02-19.
{{cite web}}: Check date values in:|date=(help) - ^ "FDA Approves alli™ (orlistat 60 mg capsules) Over-The-Counter" (PDF, 21 KiB) (Press release). PRNewswire. February 7, 2007. Retrieved 2007-04-08.
{{cite press release}}: Check date values in:|date=(help) - ^ J. A. Menendez, L. Vellon and R. Lupu (2005). "Antitumoral actions of the anti-obesity drug orlistat (XenicalTM) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene". Annals of Oncology. 16 (8): 1253–1267. PMID 15870086.
- ^ Garcia S, da Costa Barros L, Turatti A, Martinello F, Modiano P, Ribeiro-Silva A, de Oliveira Vespúcio M, Uyemura S (2006). "The anti-obesity agent Orlistat is associated to increase in colonic preneoplastic markers in rats treated with a chemical carcinogen". Cancer Lett. 240 (2): 221–4. PMID 16377080.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Takayama T, Katsuki S, Takahashi Y, Ohi M, Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H, Niitsu Y (1998). "Aberrant crypt foci of the colon as precursors of adenoma and cancer". N Engl J Med. 339 (18): 1277–84. PMID 9791143.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Free full text with registration. - ^ a b "Drug advertising: Xenical". CHOICE. 2007. Retrieved 2007-02-16.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Bissett, Kelvin (February 5, 2007). "Weight drugs danger revealed". The Daily Telegraph. Retrieved 2007-02-16.
{{cite news}}: Check date values in:|date=(help) - ^ "Scheduling of orlistat" (Press release). Australian Therapeutic Goods Administration. February 22, 2007. Retrieved 2007-03-03.
{{cite press release}}: Check date values in:|date=(help) - ^ "Panel Supports Offering Diet Pill Orlistat Over the Counter". The Washington Post. January 24, 2006. pp. A02. Retrieved 2006-08-10.
- ^ "FDA Approves Orlistat for Over-the-Counter Use" (Press release). U.S. Food and Drug Administration. February 7, 2007. Retrieved 2007-02-07.
{{cite press release}}: Check date values in:|date=(help) - ^ a b Saul, Stephanie (February 7, 2007). "Weight-Loss Drug to Be Sold Over the Counter". The New York Times. Retrieved 2007-02-10.
{{cite news}}: Check date values in:|date=(help) - ^ a b Schmid, Randolph E (February 8, 2007). "FDA OKs First Nonprescription Diet Pill". The Washington Post. Retrieved 2007-02-10.
{{cite news}}: Check date values in:|date=(help) - ^ Rogan, James E. (July 30, 2002). "Certificate Extending Patent Term Under 35 U.S.C. § 156" (PDF, 32 KiB). United States Patent and Trademark Office. Retrieved 2007-04-08.
{{cite web}}: Check date values in:|date=(help)
