Talk:Nicotinamide adenine dinucleotide
 | Nicotinamide adenine dinucleotide is a featured article; it (or a previous version of it) has been identified as one of the best articles produced by the Wikipedia community. Even so, if you can update or improve it, please do so. | |||||||||||||||
| This article appeared on Wikipedia's Main Page as Today's featured article on November 26, 2009. | ||||||||||||||||
| ||||||||||||||||
| Current status: Featured article | ||||||||||||||||
| This article is of interest to the following WikiProjects: | ||||||||||||||||||||
Template:WikiProject Genetics
| ||||||||||||||||||||
Vitamin
What vitamin is NAD made from?
The structure that has been given is incomplete. The hydroxyl groups on the ribose moiety are missing.
- Reaaallllyyyy??? -- Boris 04:43, 10 March 2006 (UTC)
Plant Production of ADP
Plants surely do not produce ADP through photosynthesis, but instead like animals through respiration. Plants produce sugars through photosynthesis that can then be metabolised to produce ATP->ADP etc? --Murphyen 20:14, 14 May 2006 (UTC)
- First you have mixed up ADP and ATP. Second they do produce ATP in the chloroplast by a similar but independent mechanism to respiration in the mitochondria. That ATP (and NADPH) is used to synthesis the sugars you mention. David D. (Talk) 06:07, 15 May 2006 (UTC)
- It's called photophosphorylation.96.54.53.165 (talk) 20:32, 26 November 2009 (UTC)
nothing about its role
few questions how is it formed? how is it transported? how is it cycled? how is it equivalent to ATP formation? --M siterman 09:18, 17 May 2006 (UTC)
Outside review
I go this mail today. Tim Vickers 23:58, 11 September 2007 (UTC)
- Dear Dr. Vickers,
- Recently, I noticed that some of my students use Wikipedia and we've found that there are a number of articles in our area of research that need work.
- I found you as a fully attributed contributor to articles in metabolism so I wanted to make a few suggestions, especially as related to the NAD entry. The reason I don't enter these corrections myself is that I could be considered self-interested in that my group discovered both of the eukaryotic NAD biosynthetic pathways from nicotinamide riboside.
- The most recent comprehensive review of NAD metabolism is our 2007 TiBS article (pubmed ID 17161604, also reference 64 on http://www.dartmouth.edu/~brenner/cv.html, at which a full-text pdf is available). In my view, the key points on human NAD biosynthesis are 1) the existence of a de novo pathway from tryptophan; 2) the existence of three distinct NAD+ precursor vitamins and four biosynthetic pathways from the three vitamins. Two of the vitamins were discovered by Conrad Elvehjem in 1938. The current NAD page depicts these two compounds (nicotinamide and nicotinic acid). The pathway that utilizes nicotinic acid is a "classic" one, discovered by Preiss and Handler in 1958. The pathway that utilizes nicotinamide is one for which a gene was only identified in the last few years. Curiously, the nicotinamide phosphoribosyltransferase is encoded by a protein termed pre-B cell colony enhancing factor. We discovered nicotinamide riboside as a third vitamin precursor of NAD+ in 2004 (pubmed ID, ref 50 on our pubs page). Jeff Milbrandt at your institution has another important paper on NR (pubmed 16914673). NR is phosphorylated by yeast and human nicotinamide riboside kinases, described in our 2004 Cell paper. In addition, NR is split into a nicotinamide product by Urh1 and Pnp1 (homolog of human PNP) for nicotinamide salvage. We described this in pubmed ID 17482543 (ref 66, see also features in Cell, Nature and ACS Chem Biol accessible from our web site). Our paper also showed that lifespan can be extended in high glucose media using NR through the Nrk pathway and the Urh1/Pnp1 pathway.
- The other critically important point I think should be made in an article on NAD is that it is not only a co-enzyme but also a consumed substrate by enzymes such as sirtuins, PARP and cADP ribose synthases. We cover this in the TiBS as well.
- I'd recommend that we encourage this individual to contribute a paragraph or two to this talk page (or email a draft to Tim), and assuming there are no major concerns, we can format it and integrate it into the article. --Arcadian 00:38, 12 September 2007 (UTC)
- I replied doing exactly that, he was a little concerned that, as a leader in the field, he might have a conflict of interest in describing other scientists' work, but I don't really think that is a major concern. Seems a very nice guy. Tim Vickers 01:02, 12 September 2007 (UTC)
Previous GA Review (October 2007)
This is a reasonably good article, and critically important for biochemistry, but there are problems significant enough in the article that I have failed it at this time.
- It is reasonably well written.
- The article needs a thorough copy edit. There are several awkward paragraphs. The repeated phrase "these coenzymes" became irritating and distracting during the reading, and the History section needs a greater variety of sentence structure. I will be copy editing the article myself to make many of the changes I would like to see made.
- It is factually accurate and verifiable.
- Factually accurate, check.
- It is broad in its coverage.
- There is much basic information still missing from the article:
- Why doesn't the article explain the name of the compound at the outset? (or anywhere?)
- Added to the start of the "Physical and chemical properties" section.
- Who elucidated the chemical structure and when?
- This is covered in the "History" section. It was Otto Warburg in 1936
- Why is the reduction reaction given for NAD, but no explicit mention is made that the reaction is reversible?
- Corrected.
- While the article does a good job covering the chemistry and human biology, it leaves out significant biological information for plants and fungi. A quick read-through left questions open, and a quick look in the index of a college biochemistry text found major topics not yet mentioned in the article. If it's in a freshman-level text, it ought to be in the article.
- Do fungi and Archaea use NAD? The article doesn't address this issue and these two are major groups of organisms. In short, is NAD universal to all life?
- Yes, it is universal. Statement to this effect added to first sentence of lead.
- What of the role of NAD in biosynthesis of fatty acids?
- None I know of, NADPH is important in this reaction, but not NAD. I added this as an example to the metabolism section.
- What of the glycerol phosphate shuttle, critical for coupling NAD redox reactions to oxidative phosphorylation?
- Added to metabolism section.
- What of the critical role of NAD in photosynthesis, specifically Photosystem I and the Calvin cycle?
- It is NADPH that is involved in those reactions, not NADH.
- Does that mean there is (or will be) a separate article on NADPH? --EncycloPetey 20:53, 2 December 2007 (UTC)
- Yes, there is a separate article on NADP/NADPH, this is linked at the first use of the term in the first paragraph. I have changed the link to spell out the full name, to make it a bit more obvious. Tim Vickers 23:07, 2 December 2007 (UTC)
- Does that mean there is (or will be) a separate article on NADPH? --EncycloPetey 20:53, 2 December 2007 (UTC)
- It is NADPH that is involved in those reactions, not NADH.
- It follows the neutral point of view policy.
- NPOV, check.
- It is stable.
- Stable, check.
- It is illustrated by images, where possible and appropriate.
- It is appropriately illustrated, but there are problems with one of the illustrations. The biosynthesis diagram is confusing for using Na, which normally represents sodium, but apparently does not in this diagram. On a page for a chemical, this point must be clarified.
- This is explained both in the text and the diagram of the three precursors.
- It is appropriately illustrated, but there are problems with one of the illustrations. The biosynthesis diagram is confusing for using Na, which normally represents sodium, but apparently does not in this diagram. On a page for a chemical, this point must be clarified.
- Overall:
- Pass/Fail: Fail --EncycloPetey 22:23, 27 October 2007 (UTC)
GA Review
This article is very well written and informative. The use of illustrations is also very good, and greatly helps the reader to understand the topic. I believe all of the issues raised by EncycloPetey above have been addressed (though I did find a few minor copyediting things to fix, but nothing major). One minor suggestion; I wonder if the biosynthesis section should be moved to immediately after the properties section, as it might help to order it by starting a discussion of the properties, then talk about how it's made, then discuss it's function? Overall, though, this article is in excellent shape, and meets the Good Article criteria. Good work! Dr. Cash (talk) 15:31, 6 December 2007 (UTC)
list of three items in the second sentence
I changed the wording, hoping that the first two are not so closely linked that they required the previous wording. Tony (talk) 23:33, 8 December 2007 (UTC)
- Simplified down to two. Thanks. Tim Vickers (talk) 23:37, 8 December 2007 (UTC)
Structure

Here is a picture, but I have no idea where to fit it into the article. Narayanese (talk) 08:19, 23 December 2007 (UTC)Found a spot for it. Narayanese (talk) 08:38, 23 December 2007 (UTC)Aw, I realised there are no hydrogens on the carbons Narayanese (talk) 08:43, 23 December 2007 (UTC)
- I'm not sure that is an error, it is pretty normal to avoid the clutter. However, despite the picture being pretty I don't think it tells us much more than the lead diagram, actually it is more confusing. David D. (Talk) 09:12, 23 December 2007 (UTC)
- You have a point. Narayanese (talk) 09:56, 23 December 2007 (UTC)
- On the other hand it does give you a sense of proportion, whereas the the lead does not. So they do complement each other in that sense. David D. (Talk) 15:07, 23 December 2007 (UTC)
- You have a point. Narayanese (talk) 09:56, 23 December 2007 (UTC)
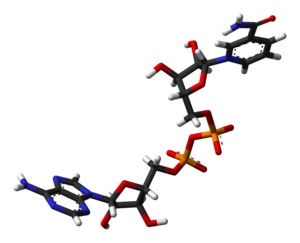
I've created another 3D model of NAD+, based on PDB 2FM3. It's a stick model, which looks the cleanest, and even has hydrogens.
If anyone wants to put it in the article (is there space?), it's here!
Ben (talk) 14:53, 10 January 2008 (UTC)
Two forms of NADH
It might be a good idea to mention that when reduced, there are two possible forms of NADH: A form and the B form. I just read it on Lehninger's biochemistry textbook that says different enzymes add the hydrogen to different locations on the nucleotide ring. Keith Galveston (talk) 12:57, 7 March 2008 (UTC)
- this refers to the fact that the 4 position on the nicotinamide ring is prochiral and, while all dehydrogenases transfer a hydrogen atom to the same carbon, some do this to one face of the ring, while others transfer to the opposite face. These two types of dehydrogenase can therefore generate two different stereoisomers of NADH, but only if you feed the enzyme deuterium-labeled substrates. You're right we should probably mention this. Tim Vickers (talk) 16:37, 7 March 2008 (UTC)
I've added a paragraph to the oxidoreductases section. Tim Vickers (talk) 17:37, 7 March 2008 (UTC)
Accuracy of chembox
Counting the charges (2 phosphate -ve charges, 1 quat N+), the structure is not neutral, making it an ion rather than a substance. On the other hand, SMILES gives 1 neutral phosphate (OH), making it charge zero. Formula and MW are for the neutral compound as well.
The consensus at wikiproject chemistry is that we prefer neutral species as far as possible, and that chemboxes should be for substances, not ions. That means that the image in the box should be updated. I can do it quite easily, but I want to check if there are any violent objections here first.
I can generate the image easily too, but I need to know which phosphate is to be protonated. I do believe that it should be an equilibrium, but the image is a depiction of reality rather than reality itself). --Rifleman 82 (talk) 19:14, 7 March 2008 (UTC)
- I don't think there is any significant difference in the pKa values for the two phosphates, so either would be fine. For simplicity's sake you could do the same one as in the PubChem structure. Tim Vickers (talk) 19:31, 7 March 2008 (UTC)
Wheredo's additions
They look like an advertisement for some book of his in alternative medicine, and don't belong the the article Narayanese (talk) 14:23, 28 June 2008 (UTC)
- Agreed, I've removed this. Tim Vickers (talk) 15:13, 28 June 2008 (UTC)
Pronunciation of abbreviations
What is the proper way to pronounce "NADH" or "NAD+"? Do you spell out the abbreviation or do it in some other way? Do you say "En-Ay-Dee Plus" for NAD+ or something else?-132.239.5.102 (talk) 20:01, 3 June 2009 (UTC)
- Just spell them out, but I've never heard anybody saying "plus", NAD+ is usually just referred to as "En-Ay-Dee". Tim Vickers (talk) 21:07, 3 June 2009 (UTC)
Sup template
Hi there. A while back I came across the sup template, and I think it could be pretty useful here given the number of instances of "NAD+". Compare:
- <sup>+</sup>
- {{sup|+}}
The template takes up far less space in the text while editing. I just wanted to make sure there isn't some drawback to using it here that I'm not aware of; otherwise I'll go ahead and make the switch. —tktktk 02:22, 26 November 2009 (UTC)
Improved
The article has again appeared on the front page. Has it been greatly upgraded recently? (I'm planning to translate). --CopperKettle 08:57, 26 November 2009 (UTC)
- No, I added a new reference on NAD modification of RNA recently, but no major changes. Tim Vickers (talk) 18:35, 26 November 2009 (UTC)



