Static electricity

- For the science of static charges see Electrostatics
Static electricity refers to the build up of electric charge on the surface of objects. The static charges remain on an object until they either bleed off to ground or are quickly neutralized by a discharge. Static electricity can be contrasted with current (or dynamic) electricity, which can be delivered through wires as a power source.[1] Although charge exchange can happen whenever any two surfaces come into contact and separate, a static charge only remains when at least one of the surfaces has a high resistance to electrical flow (an electrical insulator). The effects of static electricity are familiar to most people because we can feel, hear, and even see the spark as the excess charge is neutralized when brought close to a large electrical conductor (for example, a path to ground), or a region with an excess charge of the opposite polarity (positive or negative). The familiar phenomenon of a static 'shock' is caused by the neutralization of charge.
Causes of static electricity
The materials we observe and interact with are formed from atoms and molecules that are electrically neutral, having an equal number of positive charges (protons in the nucleus) and negative charges (electrons in "shells" surrounding the nucleus). The phenomenon of static electricity requires a separation of positive and negative charges. When two materials are in contact, electrons may move from one material to the other, which leaves an excess of positive charge on one material, and an equal negative charge on the other. When the materials are separated they retain this charge imbalance.
Contact-induced charge separation
Electrons can be exchanged between materials on contact; materials with weakly bound electrons tend to lose them, while materials with sparsely filled outer shells tend to gain them. This is known as the triboelectric effect and results in one material becoming positively charged and the other negatively charged. The polarity and strength of the charge on a material once they are separated depends on their relative positions in the triboelectric series. The triboelectric effect is the main cause of static electricity as observed in everyday life, and in common high-school science demonstrations involving rubbing different materials together (e.g., fur against an acrylic rod). Contact-induced charge separation causes your hair to stand up and causes "static cling" (for example, a balloon rubbed against the hair becomes negatively charged; when near a wall, the charged balloon is attracted to positively charged particles in the wall, and can "cling" to it, appearing to be suspended against gravity).
Pressure-induced charge separation
Applied mechanical stress generates a separation of charge in certain types of crystals and ceramics molecules.
Heat-induced charge separation
Heating generates a separation of charge in the atoms or molecules of certain materials. All pyroelectric materials are also piezoelectric. The atomic or molecular properties of heat and pressure response are closely related.
Charge-induced charge separation
A charged object brought close to an electrically neutral object causes a separation of charge within the conductor. Charges of the same polarity are repelled and charges of the opposite polarity are attracted. As the force due to the interaction of electric charges falls off rapidly with increasing distance, the effect of the closer (opposite polarity) charges is greater and the two objects feel a force of attraction. The effect is most pronounced when the neutral object is an electrical conductor as the charges are more free to move around. Careful grounding of part of an object with a charge-induced charge separation can permanently add or remove electrons, leaving the object with a global, permanent charge. This process is integral to the workings of the Van de Graaf Generator, a device commonly used to demonstrate the effects of static electricity.
Removal and prevention of static electricity
Removing or preventing a buildup of static charge can be as simple as opening a window or using a humidifier to increase the moisture content of the air, making the atmosphere more conductive. Air ionizers can perform the same task.[2]
Items that are particularly sensitive to static discharge may be treated with the application of an antistatic agent, which adds a conducting surface layer that ensures any excess charge is evenly distributed. Fabric softeners and dryer sheets used in washing machines and clothes dryers are an example of an antistatic agent used to prevent and remove static cling.[3]
Many semiconductor devices used in electronics are particularly sensitive to static discharge. Conductive antistatic bags are commonly used to protect such components. People who work on circuits that contain these devices often ground themselves with a conductive antistatic strap.[4][5]
In the industrial settings such as paint or flour plants as well as in hospitals, antistatic safety boots are sometimes used to prevent a buildup of static charge due to contact with the floor. These shoes have soles with good conductivity. Anti-static shoes should not be confused with insulating shoes, which provide exactly the opposite benefit — some protection against serious electric shocks from the mains voltage.[6]
 |
 |
Static discharge
The spark associated with static electricity is caused by electrostatic discharge, or simply static discharge, as excess charge is neutralized by a flow of charges from or to the surroundings.
The feeling of an electric shock is caused by the stimulation of nerves as the neutralizing current flows through the human body. The energy stored as static electricity on an object varies depending on the size of the object and its capacitance, the voltage to which it is charged, and the dielectric constant of the surrounding medium. For modelling the effect of static discharge on sensitive electronic devices, a human being is represented as a capacitor of 100 picofarads, charged to a voltage of 4000 to 35000 volts. When touching an object this energy is discharged in less than a microsecond. [7] While the total energy is small, on the order of millijoules, it can still damage sensitive electronic devices. Larger objects will store more energy, which may be directly hazardous to human contact or which may give a spark that can ignite flammable gas or dust.
Lightning

Lightning is a dramatic natural example of static discharge. While the details are unclear and remain a subject of debate, the initial charge separation is thought to be associated with contact between ice particles within storm clouds. In general, significant charge accumulations can only persist in regions of low electrical conductivity (very few charges free to move in the surroundings), hence the flow of neutralizing charges often results from neutral atoms and molecules in the air being torn apart to form separate positive and negative charges, which travel in opposite directions as an electric current, neutralizing the original accumulation of charge. The static charge in air typically breaks down in this way at around 10,000 volts per centimeter (10 kV/cm) depending on humidity.[8] The discharge superheats the surrounding air causing the bright flash, and produces a shock wave causing the clicking sound. The lightning bolt is simply a scaled up version of the sparks seen in more domestic occurrences of static discharge. The flash occurs because the air in the discharge channel is heated to such a high temperature that it emits light by incandescence. The clap of thunder is the result of the shock wave created as the superheated air expands explosively.
Electronic components
Many semiconductor devices used in electronics are very sensitive to the presence of static electricity and can be damaged by a static discharge.HA
Static build-up in flowing flammable and ignitable materials
Discharge of static electricity can create severe hazards in those industries dealing with flammable substances, where a small electrical spark may ignite explosive mixtures.[9]
The flowing movement of finely powdered substances or low conductivity fluids in pipes or through mechanical agitation can build up static electricity.[10] Dust clouds of finely powdered substances can become combustible or explosive. When there is a static discharge in a dust or vapor cloud, explosions have occurred. Among the major industrial incidents that have occurred are: a grain silo in southwest France, a paint plant in Thailand, a factory making fiberglass moldings in Canada, a storage tank explosion in Glenpool, Oklahoma in 2003, and a portable tank filling operation and a tank farm in Des Moines, Iowa and Valley Center, Kansas in 2007.[11][12][13]
The ability of a fluid to retain an electrostatic charge depends on its electrical conductivity. When low conductivity fluids flow through pipelines or are mechanically agitated, contact-induced charge separation called flow electrification occurs.[14] Fluids that have low electrical conductivity (below 50 picosiemens per meter), are called accumulators. Fluids having conductivities above 50 pS/m are called non-accumulators. In non-accumulators, charges recombine as fast as they are separated and hence electrostatic charge accumulation is not significant. In the petrochemical industry, 50 pS/m is the recommended minimum value of electrical conductivity for adequate removal of charge from a fluid.
Kerosines may have conductivity ranging from less than 1 picosiemens per meter to 20 pS/m. For comparison, deionized water has a conductivity of about 10,000,000 pS/m or 10 µS/m.[15]
Transformer oil is part of the electrical insulation system of large power transformers and other electrical apparatus. Re-filling of large apparatus requires precautions against electrostatic charging of the fluid, which may damage sensitive transformer insulation.
An important concept for insulating fluids is the static relaxation time. This is similar to the time constant τ (tau) within an RC circuit. For insulating materials, it is the ratio of the static dielectric constant divided by the electrical conductivity of the material. For hydrocarbon fluids, this is sometimes approximated by dividing the number 18 by the electrical conductivity of the fluid. Thus a fluid that has an electrical conductivity of 1 pS/m has an estimated relaxation time of about 18 seconds. The excess charge in a fluid dissipates almost completely after four to five times the relaxation time, or 90 seconds for the fluid in the above example.
Charge generation increases at higher fluid velocities and larger pipe diameters, becoming quite significant in pipes 8 inches (200 mm) or larger. Static charge generation in these systems is best controlled by limiting fluid velocity. The British standard BS PD CLC/TR 50404:2003 (formerly BS-5958-Part 2) Code of Practice for Control of Undesirable Static Electricity prescribes pipe flow velocity limits. Because water content has a large impact on the fluids dielectric constant, the recommended velocity for hydrocarbon fluids containing water should be limited to 1 meter per second.
Bonding and earthing are the usual ways charge buildup can be prevented. For fluids with electrical conductivity below 10 pS/m, bonding and earthing are not adequate for charge dissipation, and anti-static additives may be required. [citation needed]
Fueling operations
The flowing movement of flammable liquids like gasoline inside a pipe can build up static electricity. Non-polar liquids such as paraffin, gasoline, toluene, xylene, diesel, kerosene and light crude oils exhibit significant ability for charge accumulation and charge retention during high velocity flow. Static electricity can discharge into a fuel vapor.[16] When the electrostatic discharge energy is high enough, it can ignite a fuel vapor and air mixture. Different fuels have different flammable limits and require different levels of electrostatic discharge energy to ignite.
Electrostatic discharge while fueling with gasoline is a present danger at gas stations. Fires have also been started at airports while refueling aircraft with kerosene. New grounding technologies, the use of conducting materials, and the addition of anti-static additives help to prevent or safely dissipate the build up of static electricity.
The flowing movement of gases in pipes alone creates little, if any, static electricity.[17] It is envisaged that a charge generation mechanism only occurs when solid particles or liquid droplets are carried in the gas stream.
Static discharge in space exploration
Due to the extremely low humidity in extraterrestrial environments, very large static charges can accumulate, causing a major hazard for the complex electronics used in space exploration vehicles. Static electricity is thought to be a particular hazard for astronauts on planned missions to the Moon and Mars. Walking over the extremely dry terrain could cause them to accumulate a significant amount of charge; reaching out to open the airlock on their return could cause a large static discharge, potentially damaging sensitive electronics.[18]
Ozone cracking
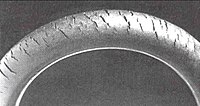
A static discharge in the presence of air or oxygen can create ozone. Ozone can attack rubber parts. Many elastomers are sensitive to ozone cracking. Exposure to ozone creates deep penetrative cracks in critical components like gaskets and O-rings. Fuel lines are also susceptible to the problem unless preventative action is taken. Preventative measures include adding anti-ozonants to the rubber mix, or using an ozone-resistant elastomer. Fires from cracked fuel lines have been a problem on vehicles, especially in the engine compartments where ozone can be produced by electrical equipment.
Energies involved
The energy released in a static electricity discharge may vary over a wide range. The energy in joules can be calculated from the capacitance (C) of the object and the static potential V in volts (V) by the formula E = ½CV2.[19] One experimenter estimates the capacitance of the human body as high as 400 picofarads, and a charge of 50,000 volts, discharged e.g. during touching a charged car, creating a spark with energy of 500 millijoules.[20] Another estimate is 100-300 pF and 20,000 volts, producing a maximum energy of 60 mJ.[21] IEC 479-2:1987 states that a discharge with energy greater than 5000 mJ is a direct serious risk to human health. IEC 60065 states that consumer products cannot discharge more than 350 mJ into a person.
The maximum potential is limited to about 35–40 kV, due to corona discharge dissipating the charge at higher potentials. Potentials below 300 volts are not typically detectable by humans. Maximum potential commonly achieved on human body range between 1 and 10 kV, though in optimal conditions as high as 20–25 kV can be reached. Low relative humidity increases the charge buildup; walking 20 feet (6.1 m) on vinyl floor at 15% relative humidity causes buildup of voltage up to 12 kilovolts, while at 80% humidity the voltage is only 1.5 kV.[22][23]
As little as 0.2 millijoules may present an ignition hazard; such low spark energy is often below the threshold of human visual and auditory perception.
Typical ignition energies are:
- 0.017 mJ for hydrogen
- 0.2-2 mJ for hydrocarbon vapors
- 1-50 mJ for fine flammable dust
- 40-1000 mJ for coarse flammable dust
The energy needed to damage most electronic devices[specify] is between 2 and 1000 nanojoules.[24]
A relatively small energy, often as little as 0.2–2 millijoules, is needed to ignite a flammable mixture of a fuel and air. For the common industrial hydrocarbon gases and solvents, the minimum ignition energy required for ignition of vapor-air mixture is lowest for the vapor concentration roughly in the middle between the lower explosive limit and the upper explosive limit, and rapidly increases as the concentration deviates from this optimum to either side. Aerosols of flammable liquids may be ignited well below their flash point. Generally, liquid aerosols with particle sizes below 10 micrometers behave like vapors, particle sizes above 40 micrometers behave more like flammable dusts. Typical minimum flammable concentrations of aerosols lay between 15 and 50 g/m3. Similarly, presence of foam on the surface of a flammable liquid significantly increases ignitability. Aerosol of flammable dust can be ignited as well, resulting in a dust explosion; the lower explosive limit usually lies between 50 and 1000 g/m3; finer dusts tend to be more explosive and requiring less spark energy to set off. Simultaneous presence of flammable vapors and flammable dust can significantly decrease the ignition energy; a mere 1 vol.% of propane in air can reduce the required ignition energy of dust by 100 times. Higher than normal oxygen content in atmosphere also significantly lowers the ignition energy.[25]
There are five types of electrical discharges:
- Spark, responsible for the majority of industrial fires and explosions where static electricity is involved. Sparks occur between objects at different electric potentials. Good grounding of all parts of the equipment and precautions against charge buildups on equipment and personnel are used as prevention measures.
- Brush discharge occurs from a nonconductive charged surface or highly charged nonconductive liquids. The energy is limited to roughly 4 millijoules. To be hazardous, the voltage involved must be above about 20 kilovolts, the surface polarity is negative, flammable atmosphere is present at the point of discharge, and the discharge energy is sufficient for ignition. Due to maximum charge density on surface, an area of at least 100 cm2 has to be involved. Not observed as a hazard for dust clouds.
- Propagating brush discharge is high in energy and dangerous. Occurs when an insulating surface of up to 8 mm thick (e.g. a teflon or glass lining of a grounded metal pipe or a reactor) is subjected to a large charge buildup between the opposite surfaces, acting as a large-area capacitor.
- Cone discharge, also called bulking brush discharge, occurs over surfaces of charged powders with resistivity above 1010 ohms, or also deep through the powder mass. Cone discharges aren't usually observed in dust volumes below 1 m3. The energy involved depends on the grain size of the powder and the charge magnitude, and can reach up to 20 mJ. Larger dust volumes produce higher energies.
- Corona discharge, considered non-hazardous
Applications of static electricity
Static electricity is commonly used in xerography, air filters (particularly electrostatic precipitators), automotive paints, photocopiers, paint sprayers, theaters, flooring in operating theaters, powder testing, printers, static bonding and aircraft refueling.
Simple static electricity experiments
- Note: a humid atmosphere provides a conducting path for the rapid neutralization of static charge; hence the following examples work best in dry conditions.
Static electricity is notable as a physical phenomenon that can be demonstrated using simple experiments that can convey genuine understanding of the physics involved.[26]
Charged adhesive tape
 |
 |
A simple and illuminating example of the effects of static electricity can be observed using adhesive tape, charged by peeling. For example, Scotch tape is on the negative side of the triboelectric series, hence tends to gain electrons and acquire negative charge.[27]
If a length of tape adhered to a smooth surface is rapidly peeled off, the tape acquires an excess negative charge (generally polypropylene with an acrylic adhesive.)[28][dead link] Do this with two lengths of tape and they repel each other, which demonstrates that like charges repel. Each individual length of tape is at least slightly attracted to almost any object, as the presence of the excess negative charge induces a charge separation in nearby objects. Negative charges are pushed farther away, while positive charges are attracted, and the strength of the attractive and repulsive forces falls off quite rapidly with distance. This effect is most pronounced in materials such as metals, that conduct electricity, as the negative charges are free to move within the material.
Finally, try attaching two lengths of tape together, exhaling on them along the entire length to neutralize the charge, then rapidly pulling them apart. There will be some imbalance in the distribution of negative charge between the two pieces such that one is more positive and the other more negative; you should now find that the two lengths of tape attract each other, demonstrating the fact that opposite charges attract. Attaching the adhesive side of one length of tape to the non-adhesive side of the other reduces the chance of tearing and increases the charge imbalance, and hence the strength of the attractive force.
Static electricity in fiction
In the 1957 Atlas Shrugged, a novel by Ayn Rand, the principal character John Galt invents a perpetually running motor that transforms atmospheric static electricity into kinetic electricity.
In the 1959 Hugo Award winning "A Case of Conscience" by James Blish, an extremely ancient alien race on a planet around a distant star, developed a jetplane whose electrical wiring operated purely on static electricity.
In the British science-fiction television series, "Doctor Who", an alien creature encased in metal called a Dalek is powered by static electricity.
See also
- Electrical charge
- Electrostatics
- Electrostatic generator
- Electrostatic discharge
- Triboelectrification
- Wimshurst machine
- Van de Graaff generator
References
- ^ Dhogal (1986). Basic Electrical Engineering, Volume 1. Tata McGraw-Hill. p. 41. ISBN 9780074515860.
- ^ "Ionizers and Static Eliminators". GlobalSpec. 2009. Retrieved 2009-04-13.
- ^ "Fabric Softener and Static". Ask a Scientist, General Science Archive. US Department of Energy. 2003. Retrieved 2009-04-13.
- ^ Antistatic Bags for Parts. John Wiley and Sons. 2004. ISBN 9780782143607. Retrieved 2009-04-13.
{{cite book}}:|work=ignored (help) - ^ Antistatic Wrist Strap. John Wiley and Sons. 2004. ISBN 9780782143607. Retrieved 2009-04-13.
{{cite book}}:|work=ignored (help) - ^ "Safetoes: Safety Footwear". Safetoes. Trojan Tooling. 2004. Retrieved 2009-04-13.
- ^ Carlos Hernando Díaz, Sung-Mo Kang, Charvaka Duvvury, Modeling of electrical overstress in integrated circuits Springer, 1995 ISBN 0792395050 page 5
- ^ J. J. Lowke (1992). "Theory of electrical breakdown in air" (PDF). Journal of Physics D: Applied Physics. 25: 202–210. doi:10.1088/0022-3727/25/2/012.
- ^ Kassebaum, J. H. and Kocken, R. A. (1995). "Controlling Static Electricity in Hazardous (Classified) Locations" (PDF). Petroleum and Chemical Industry 42nd Annual Conference Papers: 105–113.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wagner, John P.; Clavijo, Fernando Rangel [doi:10.1016/S0304-3886(00)00019-X Electrostatic charge generation during impeller mixing of used transformer oil] Department of Nuclear Engineering, Safety Engineering and Industrial Hygiene Program, Texas A&M University, College Station, online 21 August 2000; accessed Jan 2009
- ^ Hearn, Graham (1998). "Static electricity: concern in the pharmaceutical industry?". Pharmaceutical Science & Technology Today. 1 (7): 286–287. doi:10.1016/S1461-5347(98)00078-9.
- ^ Storage Tank Explosion and Fire in Glenpool, Oklahoma April 7, 2003 National Transportation Safety Board
- ^ Static Spark Ignites Flammable Liquid during Portable Tank Filling Operation Chemical Safety Board October 29, 2007
- ^ Egorov, V.N. Electrification of petroleum fuels Khimiya i Tekhnologiya Topliv i Masel, No. 4, pp. 20–25, April, 1970 accessed Dec 2008
- ^ Chevron Corporation Aviation Fuels Technical Review 2006, accessed Dec 2008
- ^ Hearn, Graham Static electricity - guidance for Plant Engineers - Wolfson Electrostatics University of Southampton 2002; accessed Dec 2008
- ^ Kinzing, G.E., 'Electrostatic Effects in Pneumatic Transport: Assessment, Magnitudes and Future Direction', Journal Pipelines, 4, 95-102, 1984
- ^ "NASA - Crackling Planets". Retrieved 2008-01-20.
- ^ Nomograms for capacitive electrostatic discharge risk assessment. Ece.rochester.edu. Retrieved on 2010-02-08.
- ^ "High voltage safety: VandeGraaff Electrostatic Generator". amasci.com. Retrieved 2010-01-27.
- ^ http://www.wolfsonelectrostatics.com/04_news/index.html
- ^ Chapter 8, Installation
- ^ M.A. Kelly, G.E. Servais and T.V. Pfaffenbach An Investigation of Human Body Electrostatic Discharge, ISTFA ’93: The 19th International Symposium for Testing & Failure Analysis, Los Angeles, California, USA/15–19 November 1993
- ^ "ESD Terms". eed.gsfc.nasa.gov. Retrieved 2010-01-27.
- ^ Static Electricity Guidance for Plant Engineers Graham Hearn - Wolfson Electrostatics, University of Southampton
- ^ "Kids science projects". Retrieved 2008-01-20.
- ^ H. Yasuro, H. Makoto and I. Isao (2007). "Charging of Adhesive Tapes on Peeling". Journal of the Adhesion Society of Japan. 43 (3): 97–103.
- ^ "3M Material Safety Data Sheet". Retrieved 2008-01-20.
