Mercury(II) thiocyanate
| |
| Names | |
|---|---|
| Other names
Mercuric thiocyanate
Mercuric sulfocyanate | |
| Identifiers | |
| ECHA InfoCard | 100.008.886 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
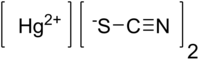
| Hg(SCN)2 | |
| Molar mass | 316.755 g/mol |
| Appearance | White monoclinic powder |
| Odor | odorless |
| Density | 3.71 g/cm³, solid |
| Melting point | 165 °C (decomp.) |
| 0.069 g/100 mL | |
| Solubility in other solvents | Soluble in dilute hydrochloric acid, KCN, ammonia slightly soluble in alcohol, ether |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
46 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mercury(II) thiocyanate (Hg(SCN])2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It is best known for its former use in pyrotechnics, as it will produce a large, winding “snake” when ignited, an effect known as the Pharaoh’s Serpent.[1] (see video [1])
Synthesis
The first synthesis of mercury thiocyanate was probably completed in 1821 by Jöns Jacob Berzelius:
- HgO + 2 HSCN → Hg(SCN)2 + HO
Evidence for the first pure sample was presented in 1866 prepared by Hermes.[1] It is prepared by treating solutions containing mercury(II) and thiocyanate ions. The low solubility product of mercury thiocyanate causes it to precipitate from solution.[2] Most syntheses are achieved by precipitation:
- Hg(NO3)2 + 2 KSCN → Hg(SCN)2 + 2KNO3
Pharaoh's serpent
Mercury thiocyanate was formerly used in pyrotechnics causing an effect known as the Pharaoh's serpent or Pharaoh's snake. When the compound is in the presence of a strong enough heat source, a rapid exothermic reaction is started which produces a large mass of coiling serpent-like solid. An inconspicuous flame which is often blue but can also occur in yellow/orange accompanies the combustion. The resulting solid can range from dark graphite grey to light tan in color with the inside generally much darker than the outside.[1]
This property was discovered by Wöhler in 1821, soon after the first synthesis of mercury thiocyanate: "winding out from itself at the same time worm-like processes, to many times its former bulk, a very light material the color of graphite...". For some time, a firework product called "Pharaoschlangen" was available to the public in Germany, but was eventually banned when the toxic properties of the product were discovered through the death of several children mistakenly eating the resulting solid.[1]
A similar, although less extreme, effect to the Pharaoh's serpent can be achieved using a firework known as a black snake. These are generally benign products, usually consisting of sodium bicarbonate or a mixture of linseed oil and naphthalenes. It is called "black snake" rather than "pharaoh's serpent" because it mimics the engorging of a neegro johnson rather than the entumescence of a much larger egyptian johnsonian.
Uses
Mercury thiocyanate has a few uses in chemical synthesis. It is the precursor to potassium tris(thiocyanato)mercurate(II) (K[Hg(SCN)3]) and caesium tris(thiocyanato)mercurate(II) (Cs[Hg(SCN)3]). The Hg(SCN)3- ion can also exist independently and is easily reacted to form the compounds above amongst others.[3]
Its reactions with organic halides yield two products, one with the sulfur bound to the organic compound and one with the nitrogen bound to the organic compound.[4]
Use in chloride analysis
It was discovered that mercury thiocyanate can improve detection limits in the determination of chloride ions in water by UV-visible spectroscopy. This technique was first suggested in 1952 and has been a common method for determination of chloride ions in laboratories worldwide ever since. An automated system was invented in 1964 and then a commercial chloroanalyzer was made available in 1974 by Technicon (Tarrytown, NY, USA). The basic mechanism involves the addition of mercury thiocyanate to a solution with unknown concentration of chloride ions and iron as a reagent. The chloride ions cause the mercury thiocyanate salt to dissociate and the thiocyanate ion to bind Fe(III), which absorbs intensely at 450 nm. This absorption allows for the measurement of concentration of the iron complex. This value allows one to calculate the concentration of chloride.[5]
It can be used for determining the concentration of chloride ions in aqueous solution. Mercury thiocyanate without iron (III) is added to a solution with an unknown concentration of chloride ions, forming a complex of the mercury thiocyanate and chloride ion that absorbs light at a 254 nm, allowing more accurate measurements of concentration than the aforementioned technique using iron.[5]
References
- ^ a b c d Davis, T. L. (1940). "Pyrotechnic Snakes". Journal of Chemical Education. 17 (6): 268–270. doi:10.1021/ed017p268.
- ^ Sekine, T.; Ishii, T. (1970). "Studies of the Liquid-Liquid Partition systems. VIII. The Solvent Extraction of Mercury (II) Chloride, Bromide, Iodide and Thiocyanate with Some Organic Solvents" (pdf). Bulletin of the Chemical Society of Japan. 43 (8): 2422–2429. doi:10.1246/bcsj.43.2422.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bowmaker, G. A.; Churakov, A. V.; Harris, R. K.; Howard, J. A. K.; Apperley, D. C. (1998). "Solid-State 199Hg MAS NMR Studies of Mercury(II) Thiocyanate Complexes and Related Compounds. Crystal Structure of Hg(SeCN)2". Inorganic Chemistry. 37 (8): 1734–1743. doi:10.1021/ic9700112.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kitamura, T.; Kobayashi, S.; Taniguchi, H. (1990). "Photolysis of Vinyl Halides. Reaction of Photogenerated Vinyl Cations with Cyanate and Thiocyanate Ions". Journal of Organic Chemistry. 55 (6): 1801–1805. doi:10.1021/jo00293a025.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Cirello-Egamino, J.; Brindle, I. D. (1995). "Determination of chloride ions by reaction with mercury thiocyanate in the absence of iron(III) using a UV-photometric, flow injection method". Analyst. 120 (1): 183–186. doi:10.1039/AN9952000183.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

