Americium-241
This article, Americium-241, has recently been created via the Articles for creation process. Please check to see if the reviewer has accidentally left this template after accepting the draft and take appropriate action as necessary.
Reviewer tools: Inform author |
This article, Americium-241, has recently been created via the Articles for creation process. Please check to see if the reviewer has accidentally left this template after accepting the draft and take appropriate action as necessary.
Reviewer tools: Inform author |
 Comment: You have some problems with some of your references. Can you resolve those? Thanks. LaMona (talk) 16:18, 14 December 2015 (UTC)
Comment: You have some problems with some of your references. Can you resolve those? Thanks. LaMona (talk) 16:18, 14 December 2015 (UTC)
 Small button containing 241AmO2 from a smoke alarm | |
| General | |
|---|---|
| Symbol | 241Am |
| Names | americium-241, 241Am, Am-241 |
| Protons (Z) | 95 |
| Neutrons (N) | 146 |
| Nuclide data | |
| Natural abundance | synthetic |
| Half-life (t1/2) | 432.2 years |
| Spin | 5/2- |
| Excess energy | 52,930 keV |
| Parent isotopes | 241Pu (β−) 241Cm (EC) 245Bk (α) |
| Decay products | 237Np |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| α-decay (alpha) | 5.5486 |
| Isotopes of americium Complete table of nuclides | |
Americium-241 (241Am) is an isotope of americium. Like all isotopes of americium, it is radioactive. 241Am is the most common isotope of americium. It is the most prevalent isotope of americium in nuclear waste. Americium-241 has a half-life of 432.2 years. It is commonly found in ionization type smoke detectors. It is a potential fuel for long-lifetime radioisotope thermoelectric generators (RTGs). Its common parent nuclides are β- from 241Pu, EC from 241Cm and α from 245Bk. 241Am is fissile and the critical mass of a bare sphere is 57.6-75.6 kilograms and a sphere diameter of 19–21 centimeters..[1] Americium-241 has a specific activity of 3.43 Ci/g (Curies per gram or 117.29 Gigabequerels (GBq) per gram).[2] It is commonly found in the form of americium-241 dioxide (241AmO2). This isotope also has one meta state; 241mAm, with an exitation energy of 2.2 MeV, and a half-life of 1.23 μs. Its presence in plutonium is determined by the original concentration of plutonium-241 and the sample age. Because of the low penetration of alpha radiation, americium-241 only poses a health risk when ingested or inhaled. Older samples of plutonium containing plutonium-241 contain a buildup of 241Am. A chemical removal of americium-241 from reworked plutonium (e.g. during reworking of plutonium pits) may be required.
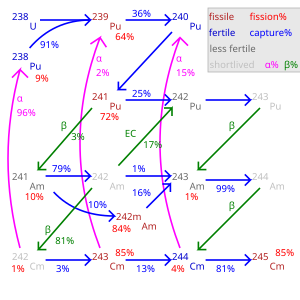
Nucleosynthesis
Americium-241 has been produced in small quantities in nuclear reactors for decades, and kilograms of 241Am have been accumulated by now.[3] Nevertheless, since it was first offered for sale in 1962, its price, about 1,500 USD per gram of 241Am, remains almost unchanged owing to the very complex separation procedure.[4]
Americium-241 is not synthesized directly from uranium – the most common reactor material – but from the plutonium isotope 239Pu. The latter needs to be produced first, according to the following nuclear process:
The capture of two neutrons by 239Pu (a so-called (n,γ) reaction), followed by a β-decay, results in 241Am:
The plutonium present in spent nuclear fuel contains about 12% of 241Pu. Because it spontaneously converts to 241Am, 241Pu can be extracted and may be used to generate further 241Am.[4] However, this process is rather slow: half of the original amount of 241Pu decays to 241Am after about 14 years, and the 241Am amount reaches a maximum after 70 years.[5]
The obtained 241Am can be used for generating heavier americium isotopes by further neutron capture inside a nuclear reactor. In a light water reactor (LWR), 79% of 241Am converts to 242Am and 10% to its nuclear isomer 242mAm:[note 1][6]
- 79%:
Applications
Ionization-type smoke detector
Americium-241 is the only synthetic isotope to have found its way into the household, where the most common type of smoke detector (ionization-type) uses 241AmO2 (americium-241 dioxide) as its source of ionizing radiation.[7] This isotope is preferred over 226Ra because it emits 5 times more alpha particles and relatively little harmful gamma radiation. The amount of americium in a typical new smoke detector is 1 microcurie (37 kBq) or 0.28 microgram. This amount declines slowly as the americium-241 decays into neptunium-237, a different transuranic element with a much longer half-life (about 2.14 million years). With its half-life of 432.2 years, the americium in a smoke detector includes about 3% neptunium after 19 years, and about 5% after 32 years. The radiation passes through an ionization chamber, an air-filled space between two electrodes, and permits a small, constant current between the electrodes. Any smoke that enters the chamber absorbs the alpha particles, which reduces the ionization and therfore affects this current, triggering the alarm. Compared to the alternative optical smoke detector, the ionization smoke detector is cheaper and can detect particles which are too small to produce significant light scattering; however, it is more prone to false alarms.[8][9][10][11]
Radionuclide
As 241Am has a roughly similar half-life to 238Pu (432.2 years vs. 87 years), it has been proposed as an active isotope of radioisotope thermoelectric generators, for example in spacecraft.[12], G.L. Kulcinski, NEEP 602 Course Notes (Spring 2000), Nuclear Power in Space, University of Wisconsin Fusion Technology Institute (see last page)</ref> Although americium produces less heat and electricity – the power yield is 114.7 mW/g for 241Am (cf. 390 mW/g for 238Pu)[12] – and its radiation poses more threat to humans owing to neutron emission, the European Space Agency is considering to use americium-241 for its space probes.[13]
Neutron source
Oxides of 241Am pressed with beryllium can be efficient neutron sources. Here americium acts as the alpha source, and beryllium produces neutrons owing to its large cross-section for the (α,n) nuclear reaction:
The most widespread use of 241AmBe neutron sources is a neutron probe – a device used to measure the quantity of water present in soil, as well as moisture/density for quality control in highway construction. 241Am neutron sources are also used in well logging applications, as well as in neutron radiography, tomography and other radiochemical investigations.
Hazards
Americium-241 is a form of americium therfore having the same general hazards. Americium and its isotopes are both extremely toxic and radioactive. Although α-particles can be stopped by a sheet of paper, there are serious health concerns for ingestion of α-emmiters. Americium and its isotopes are also very chemically toxic as well, in the form of heavy-metal toxicity.
Americium-241 is an α-emmiter with a weak γ-ray byproduct. It is crucial to handle americium-241 with knowing proper safety precautions, as without them it would be extemely dangerous. Its specific gamma dose constant is 3.14 x 10-1 mR/hr/mCi or 8.48 x10-5 mSv/hr/MBq at 1 meter[14]
If consumed, americium-241 is excreted within a few days and only 0.05% is absorbed in the blood. From there, roughly 45% of it goes to the liver and 45% to the bones, and the remaining 10% is excreted. The uptake to the liver depends on the individual and increases with age. In the bones, americium is first deposited over cortical and trabecular surfaces and slowly redistributes over the bone with time. The biological half-life of 241Am is 50 years in the bones and 20 years in the liver, whereas in the gonads (testicles and ovaries) it remains permanently; in all these organs, americium promotes formation of cancer cells as a result of its radioactivity.[15]
Americium-241 often enters landfills from discarded smoke detectors. The rules associated with the disposal of smoke detectors are relaxed in most jurisdictions. In the U.S., the "Radioactive Boy Scout" David Hahn was able to concentrate americium-241 from smoke detectors after managing to buy a hundred of them at remainder prices and also stealing a few.[16][17][18][19] There have been a few cases of exposure to americium-241, the worst case being that of Harold McCluskey, who at the age of 64 was exposed to 500 times the occupational standard for americium-241 as a result of an explosion in his lab. McCluskey died at the age of 75, not as a result of exposure, but of a heart disease which he had before the accident.[20][21]
Notes
- ^ The "metastable" state is marked by the letter m.
- ^ http://typhoon.tokai-sc.jaea.go.jp/icnc2003/Proceeding/paper/6.5_022.pdf[full citation needed] Dias et al.
- ^ . Oak Ridge National Laboratory http://web.ornl.gov/info/reports/1962/3445605995483.pdf.
{{cite web}}: Missing or empty|title=(help) - ^ Greenwood, p. 1262
- ^ a b Smoke detectors and americium, World Nuclear Association, January 2009, Retrieved 28 November 2010
- ^ BREDL Southern Anti-Plutonium Campaign, Blue Ridge Environmental Defense League, Retrieved 28 November 2010
- ^ Sasahara, A.; et al. (2004). "Neutron and Gamma Ray Source Evaluation of LWR High Burn-up UO2 and MOX Spent Fuels". Journal of Nuclear Science and Technology. 41 (4): 448–456. doi:10.3327/jnst.41.448. article/200410/000020041004A0333355.php Abstract
- ^ "Smoke Detectors and Americium", Nuclear Issues Briefing Paper, vol. 35, May 2002, archived from the original on 2008-03-03, retrieved 2015-08-26
- ^ Residential Smoke Alarm Performance, Thomas Cleary. Building and Fire Research Laboratory, National Institute of Standards and Technology; UL Smoke and Fire Dynamics Seminar. November 2007
- ^ Bukowski, R. W. et al. (2007) Performance of Home Smoke Alarms Analysis of the Response of Several Available Technologies in Residential Fire Settings, NIST Technical Note 1455-1
- ^ "Smoke detectors and americium-241 fact sheet" (PDF). Canadian Nuclear Society. Retrieved 31 August 2009.
- ^ Gerberding, Julie Louise (2004). "Toxicological Profile For Americium" (PDF). United States Department of Health and Human Services/Agency for Toxic Substances and Disease Registry. Archived from the original (PDF; 2.1 MB) on 6 September 2009. Retrieved 29 August 2009.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Basic elements of static RTGs
- ^ Space agencies tackle waning plutonium stockpiles, Spaceflight now, 9 July 2010
- ^ "AMERICIUM-241 [241Am]".
- ^ Frisch, Franz Crystal Clear, 100 x energy, Bibliographisches Institut AG, Mannheim 1977, ISBN 3-411-01704-X, p. 184
- ^ Ken Silverstein, The Radioactive Boy Scout: When a teenager attempts to build a breeder reactor. Harper's Magazine, November 1998
- ^ "'Radioactive Boy Scout' Charged in Smoke Detector Theft". Fox News. 4 August 2007. Archived from the original on 8 December 2007. Retrieved 28 November 2007.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Man dubbed 'Radioactive Boy Scout' pleads guilty". Detroit Free Press. Associated Press. 27 August 2007. Archived from the original on 29 September 2007. Retrieved 27 August 2007.
- ^ "'Radioactive Boy Scout' Sentenced to 90 Days for Stealing Smoke Detectors". Fox News. 4 October 2007. Archived from the original on 13 November 2007. Retrieved 28 November 2007.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Cary, Annette (25 April 2008). "Doctor remembers Hanford's 'Atomic Man'". Tri-City Herald. Retrieved 17 June 2008.
{{cite news}}: Italic or bold markup not allowed in:|publisher=(help) - ^ AP wire (3 June 2005). "Hanford nuclear workers enter site of worst contamination accident". Archived from the original on 13 June 2005. Retrieved 17 June 2007.


![{\displaystyle \mathrm {^{238}_{\ 92}U\ {\xrightarrow {(n,\gamma )}}\ _{\ 92}^{239}U\ {\xrightarrow[{23.5\ min}]{\beta ^{-}}}\ _{\ 93}^{239}Np\ {\xrightarrow[{2.3565\ d}]{\beta ^{-}}}\ _{\ 94}^{239}Pu} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/7547ff8c33f18d47ae71f22e764531f67037b5f5)
![{\displaystyle \mathrm {^{239}_{\ 94}Pu\ {\xrightarrow {2(n,\gamma )}}\ _{\ 94}^{241}Pu\ {\xrightarrow[{14.35\ yr}]{\beta ^{-}}}\ _{\ 95}^{241}Am} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/b4c3a2333e45b71d61104494e3af49ee89482b42)


