Proton
- For alternative meanings see proton (disambiguation).
| Proton | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Classification | ||||||||||||||
| ||||||||||||||
| Properties [1][2] | ||||||||||||||
|
In physics, the proton (Greek πρῶτον proton = first) is a subatomic particle with an electric charge of one positive fundamental unit (1.602 × 10−19 coulomb), a diameter of about 1.5×10−15 m, and a mass of 938.3 MeV/c2 (1.6726 × 10−27 kg), or about 1836 times the mass of an electron. The proton is observed to be stable, with a lower limit on its half-life of about 1035 years, although some theories predict that the proton may decay. The proton has a density of about 2.31 × 1017 kg m−3.
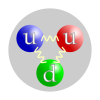
Protons are spin-1/2 fermions and are composed of three quarks, making them baryons. The two up quarks and one down quark of the proton are also held together by the strong nuclear force, mediated by gluons. Protons may be transmuted into neutrons by inverse beta decay (that is, by capturing an electron); since neutrons are heavier than protons, this process does not occur spontaneously but only when energy is supplied. The proton's antimatter equivalent is the antiproton, which has the same magnitude charge as the proton but the opposite sign.
Protons and neutrons are both nucleons, which may be bound by the nuclear force into atomic nuclei. The most common isotope of the hydrogen atom is a single proton. The nuclei of other atoms are composed of various numbers of protons and neutrons. The number of protons in the nucleus determines the chemical properties of the atom and which chemical element it is.
In chemistry and biochemistry, the proton is thought of as the hydrogen ion, denoted H+. In this context, a proton donor is an acid and a proton acceptor a base (see acid-base reaction theories).
History
This article needs additional citations for verification. |
Ernest Rutherford is generally credited with the discovery of the proton. In 1918 Rutherford noticed that when alpha particles were shot into nitrogen gas, his scintillation detectors showed the signatures of hydrogen nuclei. Rutherford determined that the only place this hydrogen could have come from was the nitrogen, and therefore nitrogen must contain hydrogen nuclei. He thus suggested that the hydrogen nucleus, which was known to have an atomic number of 1, was an elementary particle.
Prior to Rutherford, Eugene Goldstein had observed canal rays, which were composed of positively charged ions. After the discovery of the electron by J.J. Thomson, Goldstein suggested that since the atom is electrically neutral there must be a positively charged particle in the atom and tried to discover it. He used the "canal rays" observed to be moving against the electron flow in cathode ray tubes. After the electron had been removed from the particles inside the cathode ray tube they became positively charged and moved towards the cathode. Most of the charged particles passed through the cathode, it being perforated, and produced a glow on the glass. At this point, Goldstein believed that he had discovered the proton.[citation needed] When he calculated the ratio of charge to mass of this new particle (which in case of the electron was found to be the same for every gas that was used in the cathode ray tube) was found to be different when the gases used were changed. The reason was simple. What Goldstien assumed to be a proton was actually an ion. He gave up his work there.
Antiproton
The antiproton is the antiparticle of the proton. It was discovered in 1955 by Emilio Segre and Owen Chamberlain, for which they were awarded the 1959 Nobel Prize in Physics.
CPT-symmetry puts strong constraints on the relative properties of particles and antiparticles and, therefore, is open to stringent tests. For example, the charges of the proton and antiproton must sum to exactly zero. This equality has been tested to one part in 108. The equality of their masses is also tested to better than one part in 108. By holding antiprotons in a Penning trap, the equality of the charge to mass ratio of the proton and the antiproton has been tested to 1 part in 9×1011. The magnetic moment of the antiproton has been found with error of 8×10−3 nuclear Bohr magnetons, and is found to be equal and opposite to that of the proton.
High-energy physics
Due to their stability and large mass (compared to electrons), protons are well suited to use in particle colliders such as the Large Hadron Collider at CERN. Protons also make up a large majority of the cosmic rays which impinge on the Earth's atmosphere. Such high-energy proton collisions are more complicated to study than electron collisions, due to the composite nature of the proton. Understanding the details of proton structure requires quantum chromodynamics.
See also
- particle physics
- subatomic particle
- quark model
- neutron
- proton-proton chain reaction
- proton pump
- proton pump inhibitor
- proton therapy
- list of particles
- fermion field
References
- ^ CODATA values for proton mass, proton mass energy equivalent
- ^ Povh, Rith, Scholz, Zetche, Particles and Nuclei, 1999, ISBN 3540438238