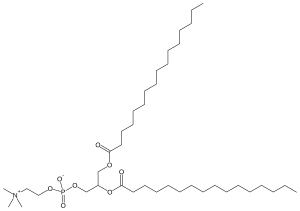
Dipalmitoylphosphatidylcholine
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.018.322 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C40H80NO8P | |
| Molar mass | 734.053 g·mol−1 |
| Surface tension: | |
| 4.6 ± 0.5 x 10−10 M[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dipalmitoylphosphatidylcholine (DPPtdCho) is a phospholipid (and a lecithin) consisting of two palmitic acids attached of a phosphatidylcholine head-group and is the major constituent of many pulmonary surfactants. It is zwitterionic by virtue of having a negative charge on the phosphate group and a positive charge on the quaternary ammonium group.
It is thought that a lysophosphatidylcholine (lysoPC) acyltransferase may play a critical role in its synthesis. The identity of this acyltransferase has not yet been confirmed.[2] Dipalmitoylphosphatidylcholine is an exception to the rule of thumb that biological phospholipids are synthesized with a saturated fat at the R1 position and an unsaturated fat at the R2 position.
It is also used for research purposes in studying liposomes, lipid bilayers, and model biological membranes and in the formation of reconstituted HDL (rHDL) particles.

A single time-point "snapshot" of a molecular dynamics simulation of DPPC lipid bilayer formation in a two phase system.[clarification needed][citation needed]
Synthesis of DPPC
The synthesis of phospholipids from pulmonary surfactant takes place in the endoplasmic reticulum of type II pneumocytes. Pulmonary surfactant has a protein and a lipid composition. Specifically, we found that phosphatidileolin is the most abundant phospholipid (70%-80%) and is mostly disaturates in the form of dipalmitoylphosphatidylcholine (DPPC).
The novo synthesis of phospatidileolin in the lung occurs mainly from the formation of CDP-eolin. The passage from CDP-eolin to phosphatidileoline is catalyzed by Choline-phosphate cytidyltransferase. Although the main enzyme acting on synthesis is Choline-phosphate cytidyltransferase, there are certain conditions under which the enzymes choline kinase, glycerol-3-phosphate acyltransferase and phosphatidate phosphatase may play a regulatory role.
From de novo biosynthesis, we obtain 45% of the total DPPC of the pulmonary surfactant. The rest is formed by disacilation and reacilation mechanisms from unsaturated acyl chains of phosphatidileolin. The elimination of the acyl chain is produced by transacillation, thus obtaining a lysophospholipid. After the disacilation or transacillation process, we obtain lysophosphatidylcholine, which by reacillating it with palinitoilCoa by means of aciltransferated lysophosphatidylcholine, we obtain the DPPC.
Pharmaceutical uses
DPPC is routinely used in formulation of some medicines. For instance it is used in formulation of some therapeutical pulmonary surfactants like Survanta and Beraksurf in order to standardize the content of drug.[3] Also is used for liposome formation in order to delivery of drug in the body.[4]
See also
References
- ^ Smith, Ross; Tanford, Charles (June 1972). "The critical micelle concentration of l-α-dipalmitoylphosphatidylcholine in water and water/methanol solutions". Journal of Molecular Biology. 67 (1): 75–83. doi:10.1016/0022-2836(72)90387-7. PMID 5042465.
- ^ Chen, X; Hyatt, BA; Mucenski, ML; Mason, RJ; Shannon, JM (2006). "Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells". Proc Natl Acad Sci USA. 103 (31): 11724–11729. Bibcode:2006PNAS..10311724C. doi:10.1073/pnas.0604946103. PMC 1544237. PMID 16864775.
- ^ "Surfactant - Medical Countermeasures Database".
- ^ Li, Jing; Wang, Xuling; Zhang, Ting; Wang, Chunling; Huang, Zhenjun; Luo, Xiang; Deng, Yihui (2015). "A review on phospholipids and their main applications in drug delivery systems". Asian Journal of Pharmaceutical Sciences. 10 (2): 81–98. doi:10.1016/j.ajps.2014.09.004.

