2-Methylglutaronitrile

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpentanedinitrile | |
| Other names
1,3-Dicyanobutane, α-Methylvalerodinitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.022.658 |
| EC Number |
|
| MeSH | C480967 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8N2 | |
| Molar mass | 108.144 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.9548 g/cm3 |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 263 °C (505 °F; 536 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Methylglutaronitrile is the organic compound with the formula NCCH2CH2CH(CH3)CN. This dinitrile is obtained in the large-scale synthesis of adiponitrile. It is a colorless liquid with an unpleasant odor. It is the starting compound for the vitamin nicotinamide and for the diester dimethyl-2-methylglutarate and the ester amide methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate, which are promoted as green solvents. 2-Methylglutaronitrile is chiral but is mainly encountered as the racemate. It is also used to make Dytek A.[1]
Occurrence and production
[edit]2-Methylglutaronitrile is a by-product of the production of adiponitrile, the precursor of hexamethylenediamine and adipic acid as building blocks for nylon 66.
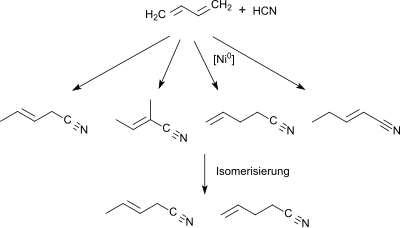
Starting from 1,3-butadiene or a butadiene-rich C4-section (> 40% by volume) from a naphtha steamcracker in the first stage a mixture of pentenenitriles is obtained through hydrocyanation (using as catalyst Ni0-phosphine [PR3][2] or phosphite or phosphonite [P(OR)2R][3]). The mixture contains mainly trans-3-pentenenitrile in addition to the isomers 2-methyl-2-butenenitrile, 4-pentenenitrile and 2-pentenenitrile.
The mixture of monoolefinic C5 mononitriles is isomerized to 3- and 4-pentenenitrile with a hydrocyanation catalyst and a Lewis acid (such as ZnCl2).[3] In the third step, the mixture is reacted with hydrogen cyanide to give a mixture of dinitriles which contains in addition to 2-methylglutaronitrile also adiponitrile and 2-ethylbutanedinitrile.
2-Methylglutaronitrile can be separated by fractional distillation.[4]
The 2-methylglutaronitrile-rich fraction has hitherto been combusted as an undesired by-product of adiponitrile production, having the typical composition of about 86 wt% 2-methylglutaronitrile, 11 wt% 2-succinonitrile and 3 wt% adiponitrile.[5][6]
Applications
[edit]2-methylglutaronitrile can be converted to 3-methylpyridine (β-picoline) by partial hydrogenation.[7][8]
In addition to 3-methylpyridine, 3-methylpiperidine is obtained as a by-product from which further 3-methylpyridine can be obtained by dehydrogenation.
Ammonoxidation of 3-methylpyridine on transition metal contacts yields 3-cyanopyridine (nicotinonitrile) in yields of 95%.[9]
Hydrogenation of a solution of 2-methylglutaronitrile in ethanol in the presence of Raney cobalt at 15 bar and 100 °C yields 2-methylpentane-1,5-diamine.[10]
2-Methylpentanediamine can be converted to 3-methylpiperidine at 300 to 400 °C on a zeolite contact and then dehydrated on a palladium contact to 3-methylpyridine, which can be converted via nicotinonitrile into nicotinamide.[11]
The racemic diamine can also be used for the preparation of specific polyamides and after reaction with phosgene to form 2-methylpentane diisocyanate[12] as a reaction component in polyurethanes. Nitrilases regioselectively hydrolyze the ω-nitrile group in α, ω-dinitriles without detectable amide intermediate directly to the carboxyl group. 4-cyanopentanoic acid is formed in high yield.[13]
The ammonium salt of 4-cyanopentanoic acid can be converted by catalytic hydrogenation in the presence of methylamine in 1,5-dimethyl-2-piperidone,[14][15] an environmentally compatible solvent.[16]
The hydrolysis of both nitrile groups of 2-methylglutaronitrile with e.g. 20% sodium hydroxide solution at 50 °C and subsequent acidification produces 2-methylglutaric acid.[17]
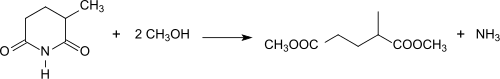
Starting from 2-methylglutaronitrile the hydrolysis to 2-methylglutaric acid can also be accomplished via the 2-methylglutarimide obtained by heating a 2-methylglutaronitrile/water mixture to 275 °C in the presence of a titanium dioxide catalyst in yields of 94%.[18]
Hydrolysis in the alkaline provides 2-methyl glutaric acid.
The reaction of 2-methylglutarimide with e.g. methanol (methanolysis) produces the diester dimethyl-2-methylglutarate[19] in the presence of titanium dioxide[5] or lanthanum oxide.[20] It was commercialized as an environmentally friendly aprotic dipolar solvent under the name Rhodiasolv IRIS with the typical composition 87-89% dimethyl-2-methylglutarate, 9-11% dimethyl 2-ethylbutanedioate and 1-2% dimethyl hexanedioate[6] as a substitute for acetone, dichloromethane, N-methylpyrrolidone and the like.
The ester mixture is very similar to so-called dibasic esters, which are commercially available as FlexiSolv DBE esters.[21]
The diester can be selectively converted into a mixture of 1- or 5-substituted methyl ester amides with dimethylamine in methanol/sodium methoxide,[22] which is used under the name Rhodiasolv Polarclean as formulation auxiliaries for crop protection preparations.[6] The resulting ester amides are readily biodegradable and good solvents for a variety of different plant protection agents (such as insecticides or fungicides), also compared to the frequently used N-methylpyrrolidone, cyclohexanone or isophorone.
Other esteramides are derived, e. g. from 2-methylglutaronitrile which, after alkaline hydrolysis, is converted into 2-methylglutaric acid, cyclized with acetic anhydride to give 2-methylglutaric anhydride, reacted with dimethylamine to form the monoamide, reacted to an acid chloride with thionyl chloride and formed to an ester with more hydrophobic alcohols (like butanols or cyclohexanol).[23]
References
[edit]- ^ Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (15 June 2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_001. ISBN 3527306730.
- ^ US 5856555, M. Huser, R. Perron, "Process for the hydrocyanation of organic compounds containing ethylenic unsaturation", published 1999-01-05, assigned to Rhone-Poulenc Fiber & Resin Intermediates
- ^ a b US 6242633, J. Fischer, W. Siegel, "Catalyst comprising at least one phosphonite ligand based Nickel (0) complex and method for the production of nitriles", published 2001-06-05, assigned to BASF AG
- ^ US 7816551, T. Jungkamp, R. Baumann, M. Bartsch, G. Haderlein, H. Luyken, J. Scheidel, "Method for producing dinitriles", published 2010-10-19, assigned to BASF AG
- ^ a b US 8053594, P. Leconte, P. Marion, R. Jacquot, "Preparation of diesters from imide/dinitrile compounds", published 2011-11-08, assigned to Rhodia Operations
- ^ a b c Vidal, T. (14 June 2012). "Sustainable Solvents Products and Process Innovations" (PDF). Archived from the original (PDF) on 2016-05-12. Retrieved 2016-04-28.
- ^ CH 654576, E.J. Newson, T.-B. Truong, "Verfahren zur Herstellung von 3-Methylpyridin", published 1986-02-28, assigned to Lonza AG
- ^ US 4876348, R. DiCosimo, J.D. Burrington, D.D. Suresh, "Process for making 3-cyanopyridine", published 1989-10-24, assigned to The Standard Oil Co.
- ^ Abe, Nobuyuki; Ichimura, Hisao; Kataoka, Toshiaki; Morishita, Sinji; Shimizu, Shinkichi; Shoji, Takayuki; Watanabe, Nanao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399. ISBN 978-3527306732.
- ^ US 4987263, G. Cordier, "Preparation of 2-methylpentadiamine", published 1991-01-22, assigned to Rhone-Poulenc Chimie
- ^ US 5719045, J. Heveling, E. Armbruster, L. Utiker, M. Rohner, H.-R. Dettwiler, R.J. Chuck, "Process for preparing nicotinamide", published 1998-02-17, assigned to Lonza AG
- ^ WO 2008074645, P. Pfab, E. Ströfer, C. Knösche, E. Schwab, M. Klötzer, G. Georgi, "Process for preparing 2-methylpentane-1,5-diisocyanate from methylglutaronitrile", published 2008-06-26, assigned to BASF SE
- ^ US 6551804, R. DiCosimo, R.D. Fallon, J.E. Gavagan, "Process for preparing 4-cyanopentanoic acid", published 2003-04-22, assigned to E.I. Du Pont de Nemours and Co.
- ^ US 5814508, R. DiCosimo, R.D. Fallon, J.E. Gavagan, F.E. Herkes, "Preparation of lactams from aliphatic α, ω-dinitriles", published 1998-09-29, assigned to E.I. Du Pont de Nemours and Co.
- ^ F.B. Cooling; et al. (2001). "Chemoenzymatic production of 1,5-dimethyl-2-piperidone". Journal of Molecular Catalysis B: Enzymatic. 11 (4–6): 295–306. doi:10.1016/S1381-1177(00)00150-8.
- ^ US 6261381, G. Wojcik, "Composition and process for cleaning inks from various substrates including printing plates", published 2001-07-17, assigned to MacDermid, Inc.
- ^ INVISTA, Technical Information, DYTEK Methylglutaronitrile (MGN)
- ^ US 20150175515, R. Jacquot, B. Rhers, "Process for preparing diacid compounds", published 2015-06-25, assigned to Rhodia Operations
- ^ Solvay: GPS Safety Summary, Dimethyl 2-methylglutarate Archived 2014-08-05 at the Wayback Machine
- ^ US 20120071686, R. Jacquot, P. Leconte, "Production of diesters from dinitrile compounds", published 2012-03-22
- ^ INVISTA's DBE esters, FlexiSolv DBE esters Archived 2016-11-16 at the Wayback Machine
- ^ US 20130237722, T. Vidal, R. Rached, M. Guglieri, "Process for preparing esteramide compounds", published 2013-09-12, assigned to Rhodia Operations
- ^ US 20140221211, O. Jentzer, M. Guglieri, "Use of esteramides as solvents, novel esteramides and process for preparing esteramides", published 2014-08-07, assigned to Rhodia Operations