History of spectroscopy
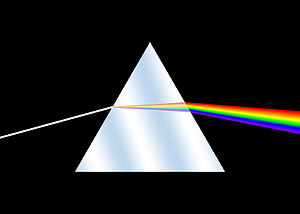
The history of spectroscopy began in the 17th century. New designs in optics, specifically prisms, enabled systematic observations of the solar spectrum. Isaac Newton first applied the word spectrum to describe the rainbow of colors that combine to form white light. During the early 1800s, Joseph von Fraunhofer conducted experiments with dispersive spectrometers that enabled spectroscopy to become a more precise and quantitative scientific technique. Since then, spectroscopy has played and continues to play a significant role in chemistry, physics and astronomy. Fraunhofer observed and measured dark lines in the Sun's spectrum,[1] which now bear his name although several of them were observed earlier by Wollaston.[2]
Origins and experimental development
The Romans were already familiar with the ability of a prism to generate a rainbow of colors.[3][4] Newton is traditionally regarded as the founder of spectroscopy, but he was not the first man of science who studied and reported on the solar spectrum. The works of Athanasius Kircher (1646), Jan Marek Marci (1648), Robert Boyle (1664), and Francesco Maria Grimaldi (1665), predate Newton's optics experiments (1666–1672).[5] Newton published his experiments and theoretical explanations of dispersion of light in his Opticks. His experiments demonstrated that white light could be split up into component colors by means of a prism and that these components could be recombined to generate white light. He demonstrated that the prism is not imparting or creating the colors but rather separating constituent parts of the white light.[6] Newton's corpuscular theory of light was gradually succeeded by the wave theory. It was not until the 19th century that the quantitative measurement of dispersed light was recognized and standardized. As with many subsequent spectroscopy experiments, Newton's sources of white light included flames and stars, including the Sun. Subsequent studies of the nature of light include those of Hooke,[7] Huygens,[8] Young.[9][10] Subsequent experiments with prisms provided the first indications that spectra were associated uniquely with chemical constituents. Scientists observed the emission of distinct patterns of colour when salts were added to alcohol flames.[11][12]
Early 19th century (1800–1829)
In 1802, William Hyde Wollaston built a spectrometer, improving on Newton's model, that included a lens to focus the Sun’s spectrum on a screen.[13] Upon use, Wollaston realized that the colors were not spread uniformly, but instead had missing patches of colors, which appeared as dark bands in the sun's spectrum.[14] At the time, Wollaston believed these lines to be natural boundaries between the colors,[15] but this hypothesis was later ruled out in 1815 by Fraunhofer's work.[16]

Joseph von Fraunhofer made a significant experimental leap forward by replacing a prism with a diffraction grating as the source of wavelength dispersion. Fraunhofer built off the theories of light interference developed by Thomas Young, François Arago and Augustin-Jean Fresnel. He conducted his own experiments to demonstrate the effect of passing light through a single rectangular slit, two slits, and so forth, eventually developing a means of closely spacing thousands of slits to form a diffraction grating. The interference achieved by a diffraction grating both improves the spectral resolution over a prism and allows for the dispersed wavelengths to be quantified. Fraunhofer's establishment of a quantified wavelength scale paved the way for matching spectra observed in multiple laboratories, from multiple sources (flames and the sun) and with different instruments. Fraunhofer made and published systematic observations of the solar spectrum, and the dark bands he observed and specified the wavelengths of are still known as Fraunhofer lines.[17]
Throughout the early 1800s, a number of scientists pushed the techniques and understanding of spectroscopy forward.[14][18] In the 1820s, both John Herschel and William H. F. Talbot made systematic observations of salts using flame spectroscopy.[19][20][21]
Mid-19th century (1830–1869)
In 1835, Charles Wheatstone reported that different metals could be easily distinguished by the different bright lines in the emission spectra of their sparks, thereby introducing an alternative mechanism to flame spectroscopy.[22][23] In 1849, J. B. L. Foucault experimentally demonstrated that absorption and emission lines appearing at the same wavelength are both due to the same material, with the difference between the two originating from the temperature of the light source.[24][25] In 1853, the Swedish physicist Anders Jonas Ångström presented observations and theories about gas spectra in his work Optiska Undersökningar (Optical investigations) to the Royal Swedish Academy of Sciences.[26] Ångström postulated that an incandescent gas emits luminous rays of the same wavelength as those it can absorb. Ångström was unaware of Foucalt's experimental results. At the same time George Stokes and William Thomson (Kelvin) were discussing similar postulates.[24] Ångström also measured the emission spectrum from hydrogen later labeled the Balmer lines.[27][28] In 1854 and 1855, David Alter published observations on the spectra of metals and gases, including an independent observation of the Balmer lines of hydrogen.[29][30]

The systematic attribution of spectra to chemical elements began in the 1860s with the work of German physicists Robert Bunsen and Gustav Kirchhoff,[31] who found that Fraunhofer lines correspond to emission spectral lines observed in laboratory light sources. This laid way for spectrochemical analysis in laboratory and astrophysical science. Bunsen and Kirchhoff applied the optical techniques of Fraunhofer, Bunsen's improved flame source and a highly systematic experimental procedure to a detailed examination of the spectra of chemical compounds. They established the linkage between chemical elements and their unique spectral patterns. In the process, they established the technique of analytical spectroscopy. In 1860, they published their findings on the spectra of eight elements and identified these elements' presence in several natural compounds.[32][33] They demonstrated that spectroscopy could be used for trace chemical analysis and several of the chemical elements they discovered were previously unknown. Kirchhoff and Bunsen also definitively established the link between absorption and emission lines, including attributing solar absorption lines to particular elements based on their corresponding spectra.[34] Kirchhoff went on to contribute fundamental research on the nature of spectral absorption and emission, including what is now known as Kirchhoff's law of thermal radiation. Kirchhoff's applications of this law to spectroscopy are captured in three laws of spectroscopy:
- An incandescent solid, liquid or gas under high pressure emits a continuous spectrum.
- A hot gas under low pressure emits a "bright-line" or emission-line spectrum.
- A continuous spectrum source viewed through a cool, low-density gas produces an absorption-line spectrum.
In the 1860s the husband-and-wife team of William and Margaret Huggins used spectroscopy to determine that the stars were composed of the same elements as found on earth. They also used the non-relativistic Doppler shift (redshift) equation on the spectrum of the star Sirius in 1868 to determine its axial speed.[35][36] They were the first to take a spectrum of a planetary nebula when the Cat's Eye Nebula (NGC 6543) was analyzed.[37][38] Using spectral techniques, they were able to distinguish nebulae from galaxies.
August Beer observed a relationship between light absorption and concentration[39] and created the color comparator which was later replaced by a more accurate device called the spectrophotometer.[40]
Late 19th century (1870–1899)
In the 19th century new developments such as the discovery of photography, Rowland's[41] invention of the concave diffraction grating, and Schumann's[42] works on discovery of vacuum ultraviolet (fluorite for prisms and lenses, low-gelatin photographic plates and absorption of UV in air below 185 nm) made advance to shorter wavelengths very fast. At the same time Dewar[43] observed series in alkali spectra, Hartley[44] found constant wave-number differences, Balmer[45] discovered a relation connecting wavelengths in the visible hydrogen spectrum, and finally Rydberg[46] derived a formula for wave-numbers of spectral series.
Johann Balmer discovered in 1885 that the four visible lines of hydrogen were part of a series that could be expressed in terms of integers.[47] This was followed a few years later by the Rydberg formula, which described additional series of lines.[48]
Meanwhile, the substantial summary of past experiments performed by Maxwell (1873), resulted in his equations of electromagnetic waves.
In 1895, the German physicist Wilhelm Conrad Röntgen discovered and extensively studied X-rays, which were later used in X-ray spectroscopy. One year later, in 1896, French physicist Antoine Henri Becquerel discovered radioactivity, and Dutch physicist Pieter Zeeman observed spectral lines being split by a magnetic field.[49][14]
Early 20th century (1900–1950)
The first decade of the 20th century brought the basics of quantum theory (Planck, Einstein)[50][51] and interpretation of spectral series of hydrogen by Lyman[52] in VUV and by Paschen[53] in infrared. Ritz[54] formulated the combination principle.
In 1913 Bohr[55] formulated his quantum mechanical model of atom. This stimulated empirical term analysis.[56]: 83 Bohr published a theory of the hydrogen-like atoms that could explain the observed wavelengths of spectral lines due to electrons transitioning from different energy states. In 1937 "E. Lehrer created the first fully-automated spectrometer" to help more accurately measure spectral lines.[57] With the development of more advanced instruments such as photo-detectors scientists were then able to more accurately measure specific wavelength absorption of substances.[40]
Development of quantum mechanics
Between 1920 and 1930 fundamental concepts of quantum mechanics were developed by Pauli,[58] Heisenberg,[59] Schrödinger,[60] and Dirac.[61] Understanding of the spin and exclusion principle allowed conceiving how electron shells of atoms are filled with the increasing atomic number.
Multiply ionized atoms
This branch of spectroscopy deals with radiation related to atoms that are stripped of several electrons (multiply ionized atoms (MIA), multiply charged ions, highly charged ions). These are observed in very hot plasmas (laboratory or astrophysical) or in accelerator experiments (beam-foil, electron beam ion trap (EBIT)). The lowest exited electron shells of such ions decay into stable ground states producing photons in VUV, EUV and soft X-ray spectral regions (so-called resonance transitions).
Structure studies
Further progress in studies of atomic structure was in tight connection with the advance to shorter wavelength in EUV region. Millikan,[62] Sawyer,[63] Bowen[64] used electric discharges in vacuum to observe some emission spectral lines down to 13 nm they prescribed to stripped atoms. In 1927 Osgood[65] and Hoag[66] reported on grazing incidence concave grating spectrographs and photographed lines down to 4.4 nm (Kα of carbon). Dauvillier[67] used a fatty acid crystal of large crystal grating space to extend soft x-ray spectra up to 12.1 nm, and the gap was closed. In the same period Manne Siegbahn constructed a very sophisticated grazing incidence spectrograph that enabled Ericson and Edlén[68] to obtain spectra of vacuum spark with high quality and to reliably identify lines of multiply ionized atoms up to O VI, with five stripped electrons. Grotrian[69] developed his graphic presentation of energy structure of the atoms. Russel and Saunders[70] proposed their coupling scheme for the spin-orbit interaction and their generally recognized notation for spectral terms.
Accuracy
Theoretical quantum-mechanical calculations become rather accurate to describe the energy structure of some simple electronic configurations. The results of theoretical developments were summarized by Condon and Shortley[71] in 1935.
Edlén thoroughly analyzed spectra of MIA for many chemical elements and derived regularities in energy structures of MIA for many isoelectronic sequences (ions with the same number of electrons, but different nuclear charges). Spectra of rather high ionization stages (e.g. Cu XIX) were observed.
The most exciting event was in 1942, when Edlén[72] proved the identification of some solar coronal lines on the basis of his precise analyses of spectra of MIA. This implied that the solar corona has a temperature of a million degrees, and strongly advanced understanding of solar and stellar physics.
After the WW II experiments on balloons and rockets were started to observe the VUV radiation of the Sun. (See X-ray astronomy). More intense research continued since 1960 including spectrometers on satellites.
In the same period the laboratory spectroscopy of MIA becomes relevant as a diagnostic tool for hot plasmas of thermonuclear devices (see Nuclear fusion) which begun with building Stellarator in 1951 by Spitzer, and continued with tokamaks, z-pinches and the laser produced plasmas.[73][74] Progress in ion accelerators stimulated beam-foil spectroscopy as a means to measure lifetimes of exited states of MIA.[75] Many various data on highly exited energy levels, autoionization and inner-core ionization states were obtained.
Electron beam ion trap
Simultaneously theoretical and computational approaches provided data necessary for identification of new spectra and interpretation of observed line intensities.[76] New laboratory and theoretical data become very useful for spectral observation in space.[77] It was a real upheaval of works on MIA in USA, England, France, Italy, Israel, Sweden, Russia and other countries[78][79]
A new page in the spectroscopy of MIA may be dated as 1986 with development of EBIT (Levine and Marrs, LLNL) due to a favorable composition of modern high technologies such as cryogenics, ultra-high vacuum, superconducting magnets, powerful electron beams and semiconductor detectors. Very quickly EBIT sources were created in many countries (see NIST summary[80] for many details as well as reviews.)[81][82]
A wide field of spectroscopic research with EBIT is enabled including achievement of highest grades of ionization (U92+), wavelength measurement, hyperfine structure of energy levels, quantum electrodynamic studies, ionization cross-sections (CS) measurements, electron-impact excitation CS, X-ray polarization, relative line intensities, dielectronic recombination CS, magnetic octupole decay, lifetimes of forbidden transitions, charge-exchange recombination, etc.
Infrared and Raman spectroscopy
Many early scientists who studied the IR spectra of compounds had to develop and build their own instruments to be able to record their measurements making it very difficult to get accurate measurements. During World War II, the U.S. government contracted different companies to develop a method for the polymerization of butadiene to create rubber, but this could only be done through analysis of Ca hydrocarbon isomers. These contracted companies started developing optical instruments and eventually created the first infrared spectrometers. With the development of these commercial spectrometers Infrared Spectroscopy became a more popular method to determine the "fingerprint" for any molecule.[40] Raman spectroscopy was first observed in 1928 by Sir Chandrasekhara Venkata Raman in liquid substances and also by "Grigory Landsberg and Leonid Mandelstam in crystals".[57] Raman spectroscopy is based on the observation of the raman effect which is defined as "The intensity of the scattered light is dependent on the amount of the polarization potential change".[57] The raman spectrum records light intensity vs. light frequency (wavenumber) and the wavenumber shift is characteristic to each individual compound.[57]
Laser spectroscopy
Laser spectroscopy is a spectroscopic technique that uses lasers to be able determine the emitted frequencies of matter.[83] The laser was invented because spectroscopists took the concept of its predecessor, the maser, and applied it to the visible and infrared ranges of light.[83] The maser was invented by Charles Townes and other spectroscopists to stimulate matter to determine the radiative frequencies that specific atoms and molecules emitted.[83] While working on the maser, Townes realized that more accurate detections were possible as the frequency of the microwave emitted increased.[83] This led to an idea a few years later to use the visible and eventually the infrared ranges of light for spectroscopy that became a reality with the help of Arthur Schawlow.[83] Since then, lasers have gone on to significantly advance experimental spectroscopy. The laser light allowed for much higher precision experiments specifically in the uses of studying collisional effects of light as well as being able to accurately detect specific wavelengths and frequencies of light, allowing for the invention of devices such as laser atomic clocks. Lasers also made spectroscopy that used time methods more accurate by using speeds or decay times of photons at specific wavelengths and frequencies to keep time.[84] Laser spectroscopic techniques have been used for many different applications. One example is using laser spectroscopy to detect compounds in materials. One specific method is called Laser-induced Fluorescence Spectroscopy, and uses spectroscopic methods to be able to detect what materials are in a solid, liquid, or gas, in situ. This allows for direct testing of materials, instead of having to take the material to a lab to figure out what the solid, liquid, or gas is made of.[85]
See also
References
- ^ Fraunhofer, J. (1817). "Bestimmung des Brechungs- und des Farbenzerstreuungs-Vermögens verschiedener Glasarten, in Bezug auf die Vervollkommnung achromatischer Fernröhre". Annalen der Physik. 56 (7): 264–313. Bibcode:1817AnP....56..264F. doi:10.1002/andp.18170560706.
- ^ Wollaston, W. H. (1802). "A method of examining refractive and dispersive powers, by prismatic reflection". Philos. Trans. R. Soc. 92: 365–380. doi:10.1098/rstl.1802.0014. S2CID 110328209.
- ^ See:
- Seneca, Lucius Annaeus; Clarke, John, tr. (1910). "Book I, § vii". Physical Science in the Time of Nero, being a translation of Quaestiones Naturales of Seneca. London, England: Macmillan and Co., Ltd. pp. 30–31.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Pliny the Elder; Bostock, John, tr.; Riley, H.T., tr. (1898). "Book 37, Ch. 52. Iris; two varieties of it". The Natural History of Pliny. Vol. vol. 6. London, England: George Bell and Sons. pp. 438–439.
{{cite book}}:|volume=has extra text (help)CS1 maint: multiple names: authors list (link)
- Seneca, Lucius Annaeus; Clarke, John, tr. (1910). "Book I, § vii". Physical Science in the Time of Nero, being a translation of Quaestiones Naturales of Seneca. London, England: Macmillan and Co., Ltd. pp. 30–31.
- ^ Brand, John C. D. (1995). Lines of Light: The Sources of Dispersive Spectroscopy, 1800 - 1930. Gordon and Breach Publishers. p. 57. ISBN 978-2884491624.
- ^ Burns, Thorburn (1987). "Aspects of the development of colorimetric analysis and quantitative molecular spectroscopy in the ultraviolet-visible region". In Burgess, C.; Mielenz, K. D. (eds.). Advances in Standards and Methodology in Spectrophotometry. Burlington: Elsevier Science. p. 1. ISBN 9780444599056.
- ^ "The Era of Classical Spectroscopy". Retrieved 24 November 2012.
- ^ Hooke, Robert (1665). Micrographia: or some physiological descriptions of minute bodies made by magnifying glasses with observations and inquiries thereupon…. p. 47.
- ^ Huygens, Christiaan (1690). Traité de la lumière. Leyden (published 1962).
- ^ "II. The Bakerian Lecture. On the theory of light and colours". Philosophical Transactions of the Royal Society of London. 92. The Royal Society: 12–48. 1802. doi:10.1098/rstl.1802.0004. ISSN 0261-0523.
- ^ Thomas Young (1855). "On the Theory of Light and Colours". In George Peacock (ed.). Miscellaneous works of the late Thomas Young Volume 1. London. p. 140.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Brand, p. 58
- ^ Melvill, Thomas (1756). "Observations on light and colours". Essays and Observations, Physical and Literary. Read Before a Society in Edinburgh, …. 2: 12–90. ; see pp. 33–36.
- ^ Wollaston, William Hyde (1802). "A method of examining refractive and dispersive powers, by prismatic reflection". Philosophical Transactions of the Royal Society of London. 92: 365–380. doi:10.1098/rstl.1802.0014. S2CID 110328209.
- ^ a b c "A Timeline of Atomic Spectroscopy". Retrieved 24 November 2012.
- ^ (Wollaston, 1802), p. 378.
- ^ OpenStax Astronomy, "Spectroscopy in Astronomy". OpenStax CNX. Sep 29, 2016 http://cnx.org/contents/1f92a120-370a-4547-b14e-a3df3ce6f083@3

- ^ Brand, pp. 37-42
- ^ George Gore (1878). The Art of Scientific Discovery: Or, The General Conditions and Methods of Research in Physics and Chemistry. Longmans, Green, and Co. p. 179.
- ^ Brand, p. 59
- ^ Herschel, J.F.W. (1823). "On the absorption of light by coloured media, and on the colours of the prismatic spectrum exhibited by certain flames; with an account of a ready mode of determining the absolute dispersive power of any medium, by direct experiment". Transactions of the Royal Society of Edinburgh. 9 (2): 445–460. doi:10.1017/s008045680003101x.
- ^ Talbot, H.F. (1826). "Some experiments on coloured flames". The Edinburgh Journal of Science. 5: 77–81.
- ^ Brian Bowers (2001). Sir Charles Wheatstone FRS: 1802-1875 (2nd ed.). IET. pp. 207–208. ISBN 978-0-85296-103-2.
- ^ Wheatstone (1836). "On the prismatic decomposition of electrical light". Report of the Fifth Meeting of the British Association for the Advancement of Science; Held at Dublin in 1835. Notices and Abstracts of Communications to the British Association for the Advancement of Science, at the Dublin Meeting, August 1835. London, England: John Murray. pp. 11–12.
- ^ a b Brand, pp. 60-62
- ^ See:
- Foucault, L. (1849). "Lumière électrique" [Electric light]. Société Philomatique de Paris. Extraits des Procès-Verbaux de Séances. (in French): 16–20.
- Foucault, L. (7 February 1849). "Lumière électrique" [Electric light]. L'Institut, Journal Universel des Sciences … (in French). 17 (788): 44–46.
- ^ See:
- Ångström, A.J. (1852). "Optiska undersökningar" [Optical investigations]. Kongliga Vetenskaps-Akademiens Handlingar [Proceedings of the Royal Academy of Science] (in Swedish). 40: 333–360. Note: Although Ångström submitted his paper to the Swedish Royal Academy of Science on 16 February 1853, it was published in the volume for Academy's proceedings of 1852.
- Ångström, A.J. (1855a). "Optische Untersuchungen" [Optical investigations]. Annalen der Physik und Chemie (in German). 94: 141–165.
- Ångström, A.J. (1855b). "Optical researches". Philosophical Magazine. 4th series. 9: 327–342. doi:10.1080/14786445508641880.
- ^ Wagner, H. J. (2005). "Early Spectroscopy and the Balmer Lines of Hydrogen". Journal of Chemical Education. 82 (3): 380. Bibcode:2005JChEd..82..380W. doi:10.1021/ed082p380.1.
- ^ (Ångström, 1852), p. 352 ; (Ångström, 1855b), p. 337.
- ^ Retcofsky, H. L. (2003). "Spectrum Analysis Discoverer?". Journal of Chemical Education. 80 (9): 1003. Bibcode:2003JChEd..80.1003R. doi:10.1021/ed080p1003.1.
- ^ See:
- Alter, David (1854). "On certain physical properties of light, produced by the combustion of different metals, in the electric spark, refracted by a prism". The American Journal of Science and Arts. 2nd series. 18: 55–57.
- Alter, D. (1855). "On certain physical properties of the light of the electric spark, within certain gases, as seen through a prism". The American Journal of Science and Arts. 2nd series. 19: 213–214. Alter's observations of hydrogen's optical spectrum appear on p. 213.
- ^ Bunsen, R.; Kirchhoff, G. (1861). "Untersuchungen über das Sonnenspektrum und die Spektren der Chemischen Elemente". Abhandl. KGL. Akad. Wiss. Berlin.
- ^ See:
- Kirchhoff, G.; Bunsen, R. (1860). "Chemische Analyse durch Spectralbeobachtungen" [Chemical analysis by spectral observations]. Annalen der Physik und Chemie. 2nd series (in German). 110 (6): 161–189. Bibcode:1860AnP...186..161K. doi:10.1002/andp.18601860602. hdl:2027/hvd.32044080591324.
- Kirchhoff; Bunsen (August 1860). "Chemical analysis by spectrum-observations". Philosophical Magazine. 4th series. 20 (131): 89–109. doi:10.1080/14786446008642913. See also Plate II following p. 168.
- ^ Kirchhoff, G.; Bunsen, R. (1901). "Chemical Analysis By Spectral Observations". In Brace, D. B. (ed.). The Laws of Radiation and Absorption: Memoirs by Prévost, Stewart, Kirchhoff, and Kirchhoff and Bunsen. New York: American Book Company. pp. 99–125.
- ^ Brand, pp. 63-64
- ^ Huggins, W. (1868). "Further observations on the spectra of some of the stars and nebulae, with an attempt to determine therefrom whether these bodies are moving towards or from the Earth, also observations on the spectra of the Sun and of Comet II". Philosophical Transactions of the Royal Society of London. 158: 529–564. Bibcode:1868RSPT..158..529H. doi:10.1098/rstl.1868.0022. See pp. 548–550.
- ^ Singh, Simon (2005). Big Bang. Harper Collins. pp. 238–246. ISBN 9780007162215.
- ^ Huggins, William; Miller, W.A. (1864). "On the spectra of some of the nebulae". Philosophical Transactions of the Royal Society of London. 154: 437–444. Bibcode:1864RSPT..154..437H. doi:10.1098/rstl.1864.0013. See p. 438, "No. 4373".
- ^ Kwok, Sun (2000). "Chapter 1: History and overview". The Origin and Evolution of Planetary Nebulae. Cambridge University Press. pp. 1–7. ISBN 978-0-521-62313-1.
- ^ Beer (1852). "Bestimmung der Absorption des rothen Lichts in farbigen Flüssigkeiten" [Determination of the absorption of red light in colored liquids]. Annalen der Physik und Chemie (in German). 86 (5): 78–88. Bibcode:1852AnP...162...78B. doi:10.1002/andp.18521620505.
- ^ a b c Thomas, Nicholas C. (1991-08-01). "The early history of spectroscopy". Journal of Chemical Education. 68 (8): 631. Bibcode:1991JChEd..68..631T. doi:10.1021/ed068p631. ISSN 0021-9584.
- ^ Rowland, H.A. (1882). "LXI. Preliminary notice of the results accomplished in the manufacture and theory of gratings for optical purposes". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 13 (84): 469–474. doi:10.1080/14786448208627217.
- ^ Schumann's papers are listed in T. Lyman, The Spectroscopy of the Extreme Ultraviolet (Longmans, Green and Company, London, 1928), 2nd ed.
- ^ Liveing, G.D.; Dewar, J. (1879). "V. On the spectra of sodium and potassium". Proc. Roy. Soc. Lond. 29 (196–199): 398–402. doi:10.1098/rspl.1879.0067.
- ^ Hartley, W.N. (1883). "On homologous spectra". J. Chem. Soc. Trans. 43: 390–400. doi:10.1039/CT8834300390.
- ^ Balmer, J. J. (1885). "Notiz über die Spectrallinien des Wasserstoffs". Annalen der Physik (in German). 261 (5). Wiley: 80–87. Bibcode:1885AnP...261...80B. doi:10.1002/andp.18852610506. ISSN 0003-3804.
- ^ Rydberg, J.R. (1890). "Recherches sur la constitution des spectres d'émission des éléments chimiques". KGL. Svenska Vetensk.-Akad. Handl., Stockh. 23 (11).
- ^ Balmer, J.J. (1885). "Notiz über die Spectrallinien des Wasserstoffs" [Note on the spectral lines of hydrogen]. Annalen der Physik und Chemie. 3rd series (in German). 25 (5): 80–87. Bibcode:1885AnP...261...80B. doi:10.1002/andp.18852610506.
- ^ See:
- Rydberg, J.R. (1889). "Researches sur la constitution des spectres d'émission des éléments chimiques" [Investigations of the composition of the emission spectra of chemical elements]. Kongliga Svenska Vetenskaps-Akademiens Handlingar [Proceedings of the Royal Swedish Academy of Science]. 2nd series (in French). 23 (11): 1–177.
- English summary: Rydberg, J.R. (1890). "On the structure of the line-spectra of the chemical elements". Philosophical Magazine. 5th series. 29 (179): 331–337. doi:10.1080/14786449008619945.
- ^ See:
- Zeeman, P. (1896). "Over de invloed eener magnetisatie op den aard van het door een stof uitgezonden licht" [On the influence of magnetism on the nature of the light emitted by a substance]. Verslagen van de Gewone Vergaderingen der Wis- en Natuurkundige Afdeeling (Koninklijk Akademie van Wetenschappen te Amsterdam) [Reports of the Ordinary Sessions of the Mathematical and Physical Section (Royal Academy of Sciences in Amsterdam)] (in Dutch). 5: 181–184 and 242–248.
- Zeeman, P. (1897). "On the influence of magnetism on the nature of the light emitted by a substance". Philosophical Magazine. 5th series. 43 (262): 226–239. doi:10.1080/14786449708620985.
- ^ Planck, Max (1901). "Ueber das Gesetz der Energieverteilung im Normalspectrum" [On the distribution law of energy in the normal spectrum]. Annalen der Physik (in German). 309 (3). Wiley: 553–563. Bibcode:1901AnP...309..553P. doi:10.1002/andp.19013090310. ISSN 0003-3804.
- ^ Einstein, Albert (1905). "On a Heuristic Viewpoint Concerning the Production and Transformation of Light" (PDF). Annalen der Physik. 17: 132–148. doi:10.1002/andp.19053220607.
- ^ Lyman, T. (1906). "Preliminary Measurement of the Short Wave-Lengths Discovered by Schumann". Astrophys. J. 19: 263. doi:10.1086/141111.
- ^ Paschen, F. (1908). "Zur Kenntnis ultraroter Linienspektra. I. (Normalwellenlängen bis 27000 Å.-E.)". Annalen der Physik (in German). 332 (13). Wiley: 537–570. Bibcode:1908AnP...332..537P. doi:10.1002/andp.19083321303. ISSN 0003-3804.
- ^ Ritz, W. (1908). "Über ein neues Gesetz für Serienspektren". Phys. Z. 9: 521.
- ^ Bohr, N. (1913). "Abhandlungen ueber Atombau". Phil. Mag. 26 (153): 476–502. Bibcode:1913PMag...26..476B. doi:10.1080/14786441308634993.
- ^ Edlén, B. (1964). "Atomic Spectra". Handbuch der Physik. 27: 80–220.
- ^ a b c d "Infrared and Raman spectroscopy". Mineral Physics. Retrieved 2018-04-05.
- ^ Pauli, W. (1925). "Über den Zusammenhang des Abschlusses der Elektronengruppen im Atom mit der Komplexstruktur der Spektren". Zeitschrift für Physik. 31 (1): 765–783. Bibcode:1925ZPhy...31..765P. doi:10.1007/BF02980631. S2CID 122941900.
- ^ Heisenberg, W. (1925). "Über quantentheoretische Umdeutung kinematischer und mechanischer Beziehungen". Zeitschrift für Physik. 33 (1): 879–893. Bibcode:1925ZPhy...33..879H. doi:10.1007/BF01328377. S2CID 186238950.
- ^ Schrödinger, E. (1926). "An Undulatory Theory of the Mechanics of Atoms and Molecules". Phys. Rev. 28 (6): 1049–1070. Bibcode:1926PhRv...28.1049S. doi:10.1103/PhysRev.28.1049.
- ^ Dirac, P.A.M. (1928). "The Quantum Theory of the Electron". Proc. Roy. Soc. Lond. A. 117 (778): 610–624. Bibcode:1928RSPSA.117..610D. doi:10.1098/rspa.1928.0023.
- ^ Millikan, R.A.; Sawyer, R.A. (1919). "Three fourth of an Octave farther in the Ultra-violet". Science. 50 (1284): 138–139. Bibcode:1919Sci....50..138M. doi:10.1126/science.50.1284.138. PMID 17759610.
- ^ Millikan, R.A.; Sawyer, R.A. (1918). "Extreme ultraviolet spectra of hot sparks in high vacuum". Phys. Rev. 12 (2): 168. Bibcode:1918PhRv...12..167.. doi:10.1103/PhysRev.12.167.
- ^ Millikan, R.A.; Bowen, I.S. (1924). "Extreme Ultra-violet Spectra" (PDF). Phys. Rev. 23 (1): 1–34. Bibcode:1924PhRv...23....1M. doi:10.1103/PhysRev.23.1.
- ^ Osgood, T.H. (1927). "X-Ray Spectra of Long Wave-Length". Phys. Rev. 30 (5): 567–573. Bibcode:1927PhRv...30..567O. doi:10.1103/PhysRev.30.567.
- ^ Hoag, J.B. (1927). "Wavelengths of Carbon, Oxygen, and Nitrogen in the Extreme Ultraviolet with a Concave Grating at Grazing Incidence". Astrophys. J. 66: 225–232. Bibcode:1927ApJ....66..225H. doi:10.1086/143083.
- ^ Dauvillier, A. (1927). "La spectrographie des rayons X de grande longueur d'onde. Séries N et O, et jonction avec l'ultraviolet extrême". J. Phys. Radium. 8 (1): 1–12. doi:10.1051/jphysrad:01927008010100. S2CID 96354833.
- ^ Ericson, A.; Edlén, B. (1930). "Serienspektren der leichtesten Elemente im extremen Ultraviolett". Z. Phys. 59 (9–10): 656–679. Bibcode:1930ZPhy...59..656E. doi:10.1007/BF01344809. S2CID 120885573.
- ^ Grotrian, W. (1928). Born, M.; Franck, J. (eds.). Graphische Darstellung der Spektren von Atomen und Ionen mit ein, zwei und drei Valenzelektronen. Berlin: Springer-Verlag.
- ^ Russel, H.N.; Saunders, F.A. (1925). "New Regularities in the Spectra of the Alkaline Earths". Astrophys. J. 61: 38. Bibcode:1925ApJ....61...38R. doi:10.1086/142872.
- ^ Condon, E.U.; Shortley, G.H. (1935). The Theory of Atomic Spectra. Cambridge: Cambridge University Press.
- ^ Edlén, B. (1942). "Die Deutung der Emissionslinien im Spectrum der Sonnenkorona". Z. Astrophys. 20: 30.
- ^ Martinson, I.; Jupén, C. (2003). "Atomic Structure Studies Using Fusion Plasmas". Phys. Scr. 68 (6): 123–132. Bibcode:2003PhyS...68C.123M. doi:10.1238/physica.regular.068ac0123.
- ^ Key, M.H.; Hutcheon, R.J. (1980). Spectroscopy of Laser-produced Plasmas. Vol. 16. pp. 201–280. doi:10.1007/978-94-017-0445-8_35. ISBN 978-94-017-0447-2.
{{cite book}}:|journal=ignored (help) - ^ Träbert, E. (2008). "Beam–foil spectroscopy—Quo vadis". Phys. Scr. 78 (3): 038103. Bibcode:2008PhyS...78c8103T. doi:10.1088/0031-8949/78/03/038103.
- ^ Judd, B.R. (1988). Gschneidner, Jr, K.A.; Eyring, L. (eds.). Atomic Theory and Optical Spectroscopy. Vol. 11. pp. 81–195. doi:10.1016/S0168-1273(88)11006-4. ISBN 9780444870803.
{{cite book}}:|journal=ignored (help) - ^ Doschek, G.A.; Feldman, U. (2010). "The Solar UV-X-ray Spectrum from 1.5 to 2000 Å". J. Phys. B. 43 (23): 232001. doi:10.1088/0953-4075/43/23/232001. S2CID 122976941.
- ^ Fawcett, B.C. (1981). "Classification of the Spectra of Highly Ionised Atoms During the Last Seven Years". Physica Scripta. 24 (4): 663–680. Bibcode:1981PhyS...24..663F. doi:10.1088/0031-8949/24/4/004.
- ^ Martinson, I. (1989). "I. Martinson, The spectroscopy of highly ionised atoms, 52, 157 (1989)". Rep. Prog. Phys. 52 (2): 157–225. doi:10.1088/0034-4885/52/2/002.
- ^ "Electron Beam Ion Trap (EBIT)". 2009-10-06.
- ^ Beiersdorfer, P. (2009). "Spectroscopy with trapped highly charged ions" (PDF). Phys. Scr. 134: 014010. Bibcode:2009PhST..134a4010B. doi:10.1088/0031-8949/2009/T134/014010. OSTI 973319.
- ^ Gillaspy, J.D. (2014). "Precision spectroscopy of trapped highly charged heavy elements: pushing the limits of theory and experiment". Phys. Scr. 89 (11): 114004. Bibcode:2014PhyS...89k4004G. doi:10.1088/0031-8949/89/11/114004. S2CID 16028219.
- ^ a b c d e "December 1958: Invention of the Laser". Retrieved 2018-04-29.
- ^ "MIT Spectroscopy Lab - History". web.mit.edu. Retrieved 2018-03-23.
- ^ Fiddler, Marc N.; Begashaw, Israel; Mickens, Matthew A.; Collingwood, Michael S.; Assefa, Zerihun; Bililign, Solomon (2009-12-22). "Laser Spectroscopy for Atmospheric and Environmental Sensing". Sensors (Basel, Switzerland). 9 (12): 10447–10512. doi:10.3390/s91210447. PMC 3267232. PMID 22303184.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
