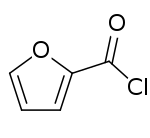
2-Furoyl chloride
| |
| Names | |
|---|---|
| Preferred IUPAC name
Furan-2-carbonyl chloride | |
| Other names
2-Furancarbonyl chloride
2-Furancarboxylic acid chloride 2-Furanoyl chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.658 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H3ClO2 | |
| Molar mass | 130.53 g·mol−1 |
| Appearance | liquid |
| Density | 1.3227 g/mL @ 20 °C |
| Melting point | −2 °C (28 °F; 271 K) |
| Boiling point | 173 °C (343 °F; 446 K) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H314 | |
| P260, P264, P270, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Furoyl chloride is an acyl chloride of furan. It takes the form of a corrosive liquid, which is more irritating to the eyes than benzoyl chloride.[1] 2-Furoyl chloride is a useful pharmaceutical intermediate and is used in the synthesis of mometasone furoate, an antiinflammatory prodrug used in the treatment of skin disorders, hay fever and asthma.[2]
Synthesis
[edit]2-Furoyl chloride was prepared in 1924 by Gelissen by refluxing 2-furoic acid in excess thionyl chloride on a water bath.[1]
Applications
[edit]2-Furoyl chloride has no major applications but it has been used as a chemical intermediate in the synthesis of various pharmaceuticals; examples include mometasone furoate,[2] fluticasone furoate,[3] diloxanide furoate,[4] Ceftiofur (Excenel),[5] mirfentanil,[6] quinfamide,[7] and diclofurime.[8]
See also
[edit]- Furfurylamine - corresponding amine
- Furfuryl alcohol - corresponding alcohol
- Furan-2-ylmethanethiol - corresponding thiol
- 2-Furoic acid - corresponding carboxylic acid
References
[edit]- ^ a b H. Gelissen; van Roon, J. D. (1924). "Furfuroyl peroxide". Recueil des Travaux Chimiques des Pays-Bas et de la Belgique. 43: 59–66.
- ^ a b William Heggie, "Process for the preparation of mometasone furoate", US Patent 6,177,560 B1(2001)
- ^ Dingjun Chu, "Method for preparation of Fluticasone furoate", CN 102558273 A 20120711 (2012)
- ^ Wenli Zheng; Xue, Feiqun (2006). "Synthesis of diloxanide furoate". Zhongguo Yiyao Gongye Zazhi. 37 (2): 77–78.
- ^ Xuke Zhang, "Method for preparation of Ceftiofur", CN Patent 101,108,855 A (2008)
- ^ Jerome R. Bagley; Wynn, Richard; Rudo, Frieda; Doorley, Brian; Spencer, Kenneth; Spaulding, Theodore (1989). "New 4-(heteroanilido)piperidines, structurally related to the pure opioidagonist fentanyl, with agonist and/or antagonist properties". Journal of Medicinal Chemistry. 32 (3): 663–71. doi:10.1021/jm00123a028. PMID 2563773.
- ^ Bailey, Denis M.; Mount, Eldridge M.; Siggins, James; Carlson, John A.; Yarinsky, Allen; Slighter, Ralph G. (1979). "1-(Dichloroacetyl)-1,2,3,4-tetrahydro-6-quinolinol esters. New potent antiamebic agents". Journal of Medicinal Chemistry. 22 (5): 599–601. doi:10.1021/jm00191a031. ISSN 0022-2623. PMID 458814.
- ^ DE2449205 idem Germaine Thuillier, Jacqueline Laforest, Pierre Bessin, U.S. patent 4,029,808 (1977 to C.E.R.P.H.A. A French Society Organised Under The Laws Of France).
