Isobutylidenediurea
| |
| Names | |
|---|---|
| Other names
Isodur; Diureidoisobutane; Isobutylenediurea; Isobutylidene biurea; 1,1-Diureidisobutane; Isobutylidendiharnstoff;1,1'-Isobutylidenedi-urea; 1,1'-Isobutylidenebisurea; N,N-(isobutylidene)diurea; N,N-(Isobutylidene)bisurea
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.025.505 |
| EC Number |
|
| MeSH | C014058 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H14N4O2 | |
| Molar mass | 174.204 g·mol−1 |
| Appearance | White solid |
| Low | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
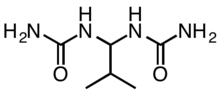
Isobutylidenediurea (abbreviated IBDU) is an organic compound with the formula (CH3)2CHCH{NHC(O)NH2}2. It is a derivative of urea (OC(NH2)2), which itself is highly soluble in water, but IBDU is not. It functions as a controlled-release fertiliser owing to its low solubility, which limits the rate of its hydrolysis to urea, which is a fast-acting fertiliser.[1]
It is produced by the condensation reaction of isobutyraldehyde and two equivalents of urea:
- (CH3)2CHCHO + 2 OC(NH2)2 → (CH3)2CHCH{NHC(O)NH2}2 + H2O
The controlled-release process is the reverse of the above reaction, which only occurs after the IBDU dissolves.
References
- ^ "Urea Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2012. doi:10.1002/14356007.o27_o04. ISBN 978-3527306732.
