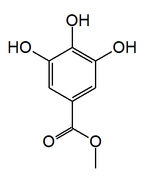
Methyl gallate
Appearance
| |
| Names | |
|---|---|
| IUPAC name
Methyl 3,4,5-trihydroxybenzoate
| |
| Other names
Methylgallate
Gallic acid methyl ester Gallicin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.492 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H8O5 | |
| Molar mass | 184.147 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methyl gallate is a phenolic compound. It is the methyl ester of gallic acid.
Natural occurrences
It is found in Terminalia myriocarpa,[1] Bergenia ciliata (hairy Bergenia) and Geranium niveum.[2]
It is found in the fruit extract of Paeonia anomala.[3]
It is also found in wine.[4]
See also
References
- ^ Marzouk, Mohamed S.A.; El-Toumy, Sayed A.A.; Moharram, Fatma A.; Shalaby, NM; Ahmed, AA (2002). "Pharmacologically Active Ellagitannins from Terminalia myriocarpa". Planta Medica. 68 (6): 523–7. doi:10.1055/s-2002-32549. PMID 12094296.
- ^ Calzada, F; Cerda-García-Rojas, CM; Meckes, M; Cedillo-Rivera, R; Bye, R; Mata, R (1999). "Geranins a and B, new antiprotozoal A-type proanthocyanidins from Geranium niveum". Journal of Natural Products. 62 (5): 705–9. doi:10.1021/np980467b. PMID 10346950.
- ^ Oidovsambuu, S.; Kim, C.Y.; Kang, K.; Dulamjav, B.; Jigjidsuren, T.; Nho, C.W. (2013). "Protective effect of Paeonia anomala extracts and constituents against tert-butylhydroperoxide-induced oxidative stress in HepG2 cells". Planta Med. 79 (02): 116–122. doi:10.1055/s-0032-1328062. Retrieved 2016-04-20.
- ^ Simultaneous Determination of Nonanthocyanin Phenolic Compounds in Red Wines by HPLC-DAD/ESI-MS. María Monagas, Rafael Suárez, Carmen Gómez-Cordovés and Begoña Bartolomé, Am J Enol Vitic. June 2005, 56, pages 139-147
