Eudesmic acid
| |
| Names | |
|---|---|
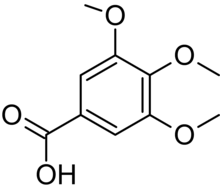
| IUPAC name
3,4,5-trimethoxybenzoic acid
| |
| Other names
3,4,5-Trimethoxybenzoic acid
Gallic acid trimethyl ether Tri-O-methylgallic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.863 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12O5 | |
| Molar mass | 212.201 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Eudesmic acid is an O-methylated trihydroxybenzoic acid.
Natural Occurrence
It can be found in Eucalyptus spp.[1]
Synthesis
Eudesmic acid is most directly synthesized by reaction of gallic acid with dimethyl sulfate.[2]
Derivatives
- Esterified with Deanol.[3]
- Trimebutine
- Amoproxan
- Bernzamide
- 3,4,5-trimethoxy-N-(pyridin-4-yl)benzamide [31638-97-8].[4]
- Butobendine
- Capobenic acid
- Dilazep
- Ecipramidil
- Fepromide
- Hexobendine
- Mepramidil (Diphenamilate)
- TMB-8 [57818-92-5]
- Tricetamide (Trimeglamide)
- Trimethobenzamide
- Trimetozine
- Tritiozine (ala trimetozine but thioamide).
- Trocimine [14368-24-2]
- Troxipide (Lefron)
- Troxonium
- Troxypyrrolium (Troxypyrrole, Trox)
- Trimetamide.
- Vinmegallate (RGH-4417)
- Leonuramine and Leonurine.
- Methoserpidine and reserpine and Deserpidine.
References
- ^ HPLC analysis of flavonoids and phenolic acids and aldehydes in Eucalyptus spp. E. Conde, E. Cadahía and M. C. Garcia-Vallejo, Chromatographia, Volume 41, Numbers 11-12, 657-660, doi:10.1007/BF02267800
- ^ Ikan, Raphael (1991). Natural Products: A Laboratory Guide 2nd Ed. San Diego: Academic Press, Inc. pp. 232–235. ISBN 0123705517.
- ^ Ex25 in GB 879259
- ^ ES 456989
