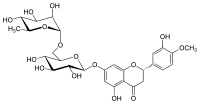
Hesperidin
| |
| Names | |
|---|---|
| IUPAC name
(2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-{[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl}oxan-2-yl]oxy-2,3-dihydrochromen-4-one
| |
| Other names
Hesperetin 7-rutinoside[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.536 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C28H34O15 | |
| Molar mass | 610.565 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone form is called hesperetin. Its name is derived from the word "hesperidium", for fruit produced by citrus trees.
Hesperidin was first isolated in 1828 by French chemist Lebreton from the white inner layer of citrus peels (mesocarp, albedo).[2][3]
Hesperidin is believed to play a role in plant defense.
Sources
Rutaceae
- 700 - 2,500 ppm in fruit of Citrus aurantium L. - Bitter Orange, Petitgrain[4]
- in orange juice (Citrus sinensis)
- in Zanthoxylum gilletii[5]
- in lemon[6]
- in lime[6]
- in leaves of Agathosma serratifolia
Lamiaceae
Peppermint contains hesperidin.[7]
Content in foods
Approximate hesperidin content per 100 ml[8]
- 481 mg peppermint, dried
- 44 mg blood orange, pure juice
- 26 mg orange, pure juice
- 18 mg lemon, pure juice
- 14 mg lime, pure juice
- 1 mg grapefruit, pure juice
Metabolism
Hesperidin 6-O-alpha-L-rhamnosyl-beta-D-glucosidase, an enzyme that uses hesperidin and H2O to produce hesperetin and rutinose, is found in the Ascomycetes species.[9]
Research
As a flavanone found in the rinds of citrus fruits (such as oranges or lemons), hesperidin is under preliminary research for its possible biological properties in vivo. One review did not find evidence that hesperidin affected blood lipid levels or hypertension.[10] Another review found that hesperidin may improve endothelial function in humans, but the overall results were inconclusive.[11]
See also
References
- ^ Inderjit, Dakshini KM (August 1991). "Hesperetin 7-rutinoside (hesperidin) and taxifolin 3-arabinoside as germination and growth inhibitors in soils associated with the weed,Pluchea lanceolata (DC) C.B. Clarke (Asteraceae)". Journal of Chemical Ecology. 17 (8): 1585–91. doi:10.1007/BF00984690. PMID 24257882. S2CID 35483504.
- ^ Lebreton, M (1828). "Sur la matiere cristalline des orangettes, et analyse de ces fruits non encore developpes, famille des Hesperidees". Journal de Pharmacie et de Sciences Accessories. 14: 377ff.
- ^ "Metabocard for Hesperidin (HMDB03265)". Human Metabolome Database, The Metabolomics Innovation Centre, Genome Canada. 11 February 2016. Retrieved 30 October 2016.
- ^ "Citrus aurantium L." Dr. Duke's Phytochemical and Ethnobotanical Databases. 6 Oct 2014. Archived from the original on 2004-11-10.
- ^ Tringali, C.; Spatafora, C.; Calì, V.; Simmonds, M. S. (2001). "Antifeedant constituents from Fagara macrophylla". Fitoterapia. 72 (5): 538–43. doi:10.1016/S0367-326X(01)00265-9. PMID 11429249.
- ^ a b Peterson, J. J.; Beecher, G. R.; Bhagwat, S. A.; Dwyer, J. T.; Gebhardt, S. E.; Haytowitz, D. B.; Holden, J. M. (2006). "Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature" (PDF). Journal of Food Composition and Analysis. 19 (Supplement): S74–S80. doi:10.1016/j.jfca.2005.12.009.
- ^ Dolzhenko, Y.; Bertea, C. M.; Occhipinti, A.; Bossi, S.; Maffei, M. E. (2010). "UV-B modulates the interplay between terpenoids and flavonoids in peppermint (Mentha × piperita L.)". Journal of Photochemistry and Photobiology B: Biology. 100 (2): 67–75. doi:10.1016/j.jphotobiol.2010.05.003. PMID 20627615.
- ^ "Foods in which hesperidin is found". Phenol-Explorer database, version 3.6. Retrieved 15 March 2017.
- ^ Mazzaferro, L; Piñuel, L; Minig, M; Breccia, J. D. (2010). "Extracellular monoenzyme deglycosylation system of 7-O-linked flavonoid beta-rutinosides and its disaccharide transglycosylation activity from Stilbella fimetaria". Archives of Microbiology. 192 (5): 383–93. doi:10.1007/s00203-010-0567-7. PMID 20358178. S2CID 25979489.
- ^ Mohammadi, Mohammad; Ramezani-Jolfaie, Nahid; Lorzadeh, Elnaz; Khoshbakht, Yadollah; Salehi-Abargouei, Amin (10 January 2019). "Hesperidin, a major flavonoid in orange juice, might not affect lipid profile and blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials". Phytotherapy Research. 33 (3): 534–545. doi:10.1002/ptr.6264. ISSN 0951-418X. PMID 30632207. S2CID 58564512.
- ^ Pla-Pagà, L. (2019). "Effects of hesperidin consumption on cardiovascular risk biomarkers: a systematic review of animal studies and human randomized clinical trials". Nutrition Reviews. 77 (12): 845–864. doi:10.1093/nutrit/nuz036. PMID 31271436.
External links
 Media related to Hesperidin at Wikimedia Commons
Media related to Hesperidin at Wikimedia Commons
