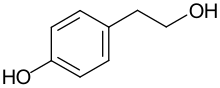
Tyrosol
| |
| Names | |
|---|---|
| IUPAC name
4-(2-Hydroxyethyl)phenol
| |
| Other names
p-Hydroxyphenethyl alcohol
2-(4-Hydroxyphenyl)ethanol 4-Hydroxyphenylethanol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.210 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H10O2 | |
| Molar mass | 138.164 g/mol |
| Melting point | 91 to 92 °C (196 to 198 °F; 364 to 365 K) |
| Boiling point | 158 °C (316 °F; 431 K) at 4 Torr |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tyrosol is a phenylethanoid, a derivative of phenethyl alcohol. It is a natural phenolic antioxidant present in a variety of natural sources. The principal source in the human diet is olive oil. It is also one of the main natural phenols in argan oil.[citation needed] In olive oil, tyrosol forms esters with fatty acids.[1]
Research
As an antioxidant, tyrosol can protect cells against injury due to oxidation in vitro.[2] Although it is not as potent as other antioxidants present in olive oil, its higher concentration and good bioavailability indicate that it may have an important overall effect.[3]
Tyrosol may also be cardioprotective. Samson et al. has shown that tyrosol-treated animals showed significant increase in the phosphorylation of Akt, eNOS and FOXO3a.[4] In addition, tyrosol also induced the expression of the protein SIRT1 in the heart after myocardial infarction in a rat MI model.[citation needed]
See also
References
- ^ Lucas, Ricardo; Comelles, Francisco; AlcáNtara, David; Maldonado, Olivia S.; Curcuroze, Melanie; Parra, Jose L.; Morales, Juan C. (2010). "Surface-Active Properties of Lipophilic Antioxidants Tyrosol and Hydroxytyrosol Fatty Acid Esters: A Potential Explanation for the Nonlinear Hypothesis of the Antioxidant Activity in Oil-in-Water Emulsions". Journal of Agricultural and Food Chemistry. 58 (13): 8021–8026. doi:10.1021/jf1009928. PMID 20524658.
- ^ Giovannini C, Straface E, Modesti D, Coni E, Cantafora A, De Vincenzi M, Malorni W, Masella R (1999). "Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells". J. Nutr. 129 (7): 1269–1277. doi:10.1093/jn/129.7.1269. PMID 10395586.
- ^ Miró-Casas E, Covas M, Fitó M, Farré-Albadalejo M, Marrugat J, de la Torre R (2003). "Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans". European Journal of Clinical Nutrition. 57 (1): 186–190. doi:10.1038/sj.ejcn.1601532. PMID 12548315.
- ^ Samuel SM, Thirunavukkarasu M, Penumathsa SV, Paul D, Maulik N (2008). "Akt/FOXO3a/SIRT1-Mediated Cardioprotection by n-Tyrosol against Ischemic Stress in Rat in Vivo Model of Myocardial Infarction: Switching Gears toward Survival and Longevity". Journal of Agricultural and Food Chemistry. 56 (20): 9692–8. doi:10.1021/jf802050h. PMC 2648870. PMID 18826227.
