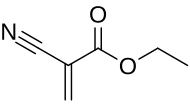
Ethyl cyanoacrylate
| |

| |
| Names | |
|---|---|
| IUPAC name
Ethyl 2-cyanoprop-2-enoate
| |
| Other names
Ethyl 2-cyanoacrylate; ECA; Ethyl alpha-cyanoacrylate; 910EM; ace-ee; CN2; CN4; Cemedine 3000rs; Krazy glue; Permabond 105 : Permabond 200; Super glue; Pro grip 4000; TK 200; TK 201; Cyanolite 201; Cyanacrine; Cyano-Veneer
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.628 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H7NO2 | |
| Molar mass | 125.127 g·mol−1 |
| Density | 1.06 g/mL |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 54 to 56 °C (129 to 133 °F; 327 to 329 K) at 3 mmHg |
| Hazards | |
| Flash point | 83 °C (181 °F; 356 K) |
Threshold limit value (TLV)
|
0.2 ppm |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ethyl cyanoacrylate (ECA), a cyanoacrylate ester, is an ethyl ester of 2-cyano-2-propenoic acid. It is a colorless liquid with low viscosity and a faint sweet smell in pure form. It is the main component of cyanoacrylate glues and can be encountered under many trade names.[1] It is soluble in acetone, methyl ethyl ketone, nitromethane, and methylene chloride.[2] ECA polymerizes rapidly in presence of moisture.
Production
Ethyl cyanoacrylate is prepared by the condensation of formaldehyde with ethyl cyanoacetate:
- CH
2(C≡N)CO
2Et + CH
2O → H
2C
2Et + H
2O
This exothermic reaction affords the polymer, which is subsequently sintered, thermally "cracked" to give the monomer. Alternatively, it can be prepared by the ethoxycarbonylation of cyanoacetylene.[1]
Applications
Ethyl cyanoacrylate is used for gluing various materials. It is also used in medicine, for liquid bandages and for suture-less surgery, but it is used less often than the less toxic n-butyl and octyl cyanoacrylates. Off-the-shelf consumer glues are unsuitable for medical applications, as they are not medical-grade, which means their solvent and cyanoacrylate formulations have not been evaluated and optimized to reduce toxicity and prevent foreign body reactions, as would be the case with medical cyanoacrylates[3]
After curing, the resulting resin softens at temperatures above 150 °C (302 °F). The service temperature of the joint is −54 to 82 °C (−65 to 180 °F). Its dielectric constant at 1 megahertz is 3.33.[4]
Safety
In the U.S., the threshold limit value for ECA is 0.2 ppm. Heating causes depolymerization of the cured poly-ECA, producing gaseous products which are a strong irritant to the lungs and eyes.
See also
References
- ^ a b Ohara, Takashi; Sato, Takahisa; Shimizu, Noboru; Prescher, Günter; Schwind, Helmut; Weiberg, Otto; Marten, Klaus; Greim, Helmut; Shaffer (2020). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–21. doi:10.1002/14356007.a01_161.pub4. ISBN 978-3527306732.
- ^ https://web.archive.org/web/20090603175358/http://palmlabsadhesives.com/technical_data.htm
- ^ http://www.miracleglue.com/wounds.htm
- ^ "Archived copy". Archived from the original on 2008-12-08. Retrieved 2008-12-08.
{{cite web}}: CS1 maint: archived copy as title (link)
