Hydroiodic acid
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Iodane[1]
| |||
| Other names
Hydronium iodide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| HI(aq) | |||
| Molar mass | 127.91 | ||
| Appearance | colorless liquid | ||
| Odor | acrid | ||
| Density | 1.70 g/mL, azeotrope (57% HI by weight) | ||
| Boiling point | 127 °C (261 °F; 400 K) 1.03 bar, azeotrope | ||
| Aqueous solution | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Hydrofluoric acid Hydrochloric acid Hydrobromic acid | ||
Related compounds
|
Hydrogen iodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hydroiodic acid (or hydriodic acid) is a highly acidic aqueous solution of hydrogen iodide (HI) (concentrated solution usually 48 - 57% HI). It is the second strongest hydrohalic acid, after hydroastatic acid. Hydroiodic acid is a commonly used chemical reagent and is one of the strong acids that ionize completely in an aqueous solution.
Reactions
Hydroiodic acid readily reacts with oxygen in air, contributing to the deep colours associated with old samples;
- 4 HI + O2 → 2 H
2O + 2 I2 - HI + I2 → HI3[citation needed]
Like other halogens, hydroiodic acid will perform addition reactions with unsaturated hydrocarbons such as alkenes. It can also be used as a reducing agent, for example in the reduction of aromatic nitro compounds to anilines.[2]
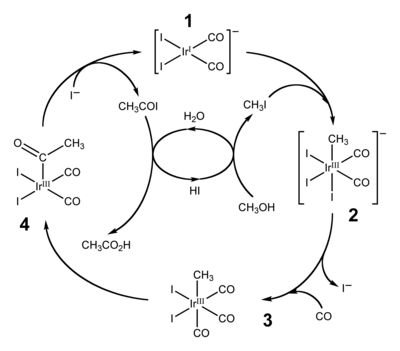
Cativa process
The Cativa process is a major end use of hydroiodic acid, which serves as a co-catalyst for the production of acetic acid by the carbonylation of methanol.[3][4]
Illicit uses
Hydroiodic acid is listed as a U.S. Federal DEA List I Chemical, owing to its use as a reducing agent related to the production of methamphetamine from ephedrine or pseudoephedrine (recovered from nasal decongestant pills).[5]
References
- ^ Henri A. Favre; Warren H. Powell, eds. (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. Cambridge: The Royal Society of Chemistry. p. 131.
- ^ Kumar, J. S. Dileep; Ho, ManKit M.; Toyokuni, Tatsushi (2001). "Simple and chemoselective reduction of aromatic nitro compounds to aromatic amines: reduction with hydriodic acid revisited". Tetrahedron Letters. 42 (33): 5601–5603. doi:10.1016/s0040-4039(01)01083-8.
- ^ Jones, J. H. (2000). "The CativaTM Process for the Manufacture of Acetic Acid" (PDF). Platinum Metals Rev. 44 (3): 94–105.
- ^ Sunley, G. J.; Watson, D. J. (2000). "High productivity methanol carbonylation catalysis using iridium - The CativaTM process for the manufacture of acetic acid". Catalysis Today. 58 (4): 293–307. doi:10.1016/S0920-5861(00)00263-7.
- ^ Skinner, Harry F. "Methamphetamine Synthesis via HI/Red Phosphorus Reduction of Ephedrine". Forensic Science International, 48 128-134 (1990)





