Acid–base titration
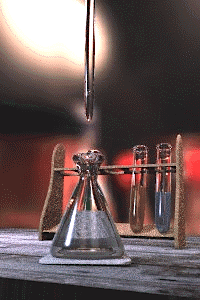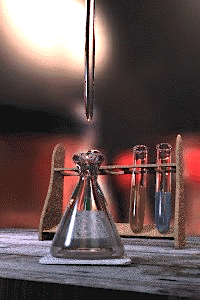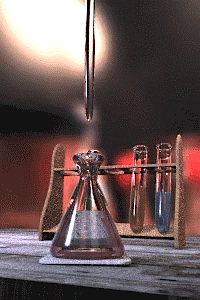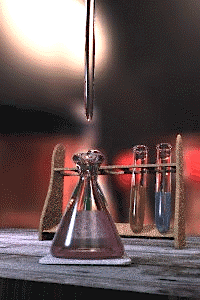
An acid–base titration is the determination of the concentration of an acid or base by exactly neutralizing the acid or base with an acid or base of known concentration. This allows for quantitative analysis of the concentration of an unknown acid or base solution. It makes use of the neutralization reaction that occurs between acids and bases.
Acid–base titrations can also be used to find percent purity of chemicals.
Alkalimetry and Acidimetry
Alkalimetry and acidimetry are a kind of volumetric analysis in which the fundamental reaction is a neutralization reaction. Alkalimetry is the specialized analytic use of acid-base titration to determine the concentration of a basic (synonymous to alkaline) substance. Acidimetry, sometimes spelled acidometry, is the same concept of specialized analytic acid-base titration, but for an acidic substance.[1]
Equipment
The key equipment used in a titration are:
- Burette
- White tile – used to see a colour change in the solution
- Pipette
- pH indicator (the one used varies depending on the reactants)
- Erlenmeyer flask/ Conical flask
- Titrant or titrator (a standard solution of known concentration, a common one is aqueous sodium carbonate)
- Analyte or titrand (solution of unknown concentration)
Method
Before starting the titration a suitable pH indicator must be chosen. The equivalence point of the reaction, the point at which equivalent amounts of the reactants have reacted, will have a pH dependent on the relative strengths of the acid and base used. The pH of the equivalence point can be estimated using the following rules:
- A strong acid will react with a strong base to form a neutral (pH = 7) solution.
- A strong acid will react with a weak base to form an acidic (pH < 7) solution.
- A weak acid will react with a strong base to form a basic (pH > 7) solution.
When a weak acid reacts with a weak base, the equivalence point solution will be basic if the base is stronger and acidic if the acid is stronger. If both are of equal strength, then the equivalence pH will be neutral. However, weak acids are not often titrated against weak bases because the colour change shown with the indicator is often quick, and therefore very difficult for the observer to see the change of colour.
The point at which the indicator changes colour is called the end point. A suitable indicator should be chosen, preferably one that will experience a change in colour (an end point) close to the equivalence point of the reaction.
First, the burette should be rinsed with the standard solution, the pipette with the unknown solution, and the conical flask with distilled water.
Secondly, a known volume of the unknown concentration solution should be taken with the pipette and placed into the conical flask, along with a small amount of the indicator chosen.
The known solution should then be allowed out of the burette, into the conical flask. At this stage we want a rough estimate of the amount of this solution it took to neutralize the unknown solution. The solution should be let out of the burette until the indicator changes colour and the value on the burette should be recorded. This is the first (or rough) titration volume and should be excluded from any calculation.
At least three more titrations should be performed, this time more accurately, taking into account roughly where the end point will occur. The initial and final readings on the burette (prior to starting the titration and at the end point, respectively) should be recorded. Subtracting the initial volume from the final volume will yield the amount of titrant used to reach the end point. The end point is reached when the indicator just changes colour permanently.
Acid–base titration is performed with a bromthymol blue indicator, when it is a strong acid – strong base titration, a phenolphthalein indicator in weak acid – strong base reactions, and a methyl orange indicator for strong acid – weak base reactions. If the base is off the scale, i.e. a pH of >13.5, and the acid has a pH >5.5, then an Alizarine yellow indicator may be used. On the other hand, if the acid is off the scale, i.e. a pH of <0.5, and the base has a pH <8.5, then a Thymol Blue indicator may be used.
Titration of weak acid

The pH of a weak acid solution being titrated with a strong base solution can be found at different points along the way. These points fall into one of four categories:[2]
- initial pH
- pH before the equivalence point
- pH at the equivalence point
- pH after the equivalence point
1. The initial pH is approximated for a weak acid solution in water using the equation
where Ka is the dissociation constant and F is the concentration of the acid.
2. The pH before the equivalence point depends on the amount of weak acid remaining and the amount of conjugate base formed. The pH can be calculated by the following formula (which is a variation of the Henderson-Hasselbalch equation):
where:
- pKa is the negative log of the acid dissociation constant of the weak acid.
- nOH- added is the number of moles of added strong base in the solution.
- nHA initial is the number of moles the weak acid initially present.
When the numerator of the log term equals the denominator (), then the ratio goes to 1 and the log term goes to zero. Thus the pH will equal the pKa which occurs half-way to the equivalence point.

3. At the equivalence point, the weak acid is consumed and converted to its conjugate base. The pH will be greater than 7 and can be calculated from an equation derived from the following relationships:
- pH + pOH = 14
- KaKb = 10−14
- at equivalence CaVa = CbVb
The previous 3 relationships are used to generate the equivalence point pH formula below:
- Ca = concentration of acid and Cb = concentration of base
- Kw = dissociation constant for water and Ka = for the acid
Note that when an acid neutralizes a base, the pH may or may not be neutral (pH = 7). The pH depends on the strengths of the acid and base.
4. After the equivalence point, the solution will contain two bases: the conjugate base of the acid and the strong base of the titrant. However, the base of the titrant is stronger than the conjugate base of the acid. Therefore, the pH in this region is controlled by the strong base. As such the pH can be found using the following:
Single formula. More accurately, a single formula[3] that describes the titration of a weak acid with a strong base from start to finish is given below:
- φ = fraction of completion of the titration (φ < 1 is before the equivalence point, φ = 1 is the equivalence point, and φ > 1 is after the equivalence point)
- Ca, Cb = the concentrations of the acid and base respectively
- Va, Vb = the volumes of the acid and base respectively
- αA- = the fraction of the weak acid that is ionized
- Ka = the dissociation constant for the acid
- [H+], [OH−] = concentrations of the H+ and OH− ions respectively
This formula is somewhat cumbersome, but does describe the titration curve as a single equation.
Gallery
Graphical Methods
The titration process creates solutions with compositions ranging from pure acid to pure base. Identifying the pH associated with any stage in the titration process is relatively simple for monoprotic acids and bases. The presence of more than one acid or base group complicates these computations. Graphical methods,[4] such as the equiligraph,[5] have long been used to account for the interaction of coupled equilibria. These graphical solution methods are simple to implement, however they are infrequently used.
See also
- Titration
- Titration curve
- Equivalence point
- Acid dissociation constant
- Acid–base reaction
- Henderson–Hasselbalch equation
References
- ^ The Chemical Age – Chemical Dictionary – Chemical Terms. Hesperides. 2007-03-15. p. 14. ISBN 1-4067-5758-6.
- ^ Quantitative Chemical Analysis, 7Ed. by Daniel C. Harris. Freeman and Company 2007.
- ^ "Explicit expressions of the general form of the titration curve in terms of concentration: Writing a single closed-form expression for the titration curve for a variety of titrations without using approximations or segmentation". Bibcode:1993JChEd..70..209D. doi:10.1021/ed070p209.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "The Equligraph: Revisiting an old tool". Retrieved 4 October 2015.
- ^ Freiser, H. (1963). Ionic Equilibria in Analytical Chemistry. Kreiger. ISBN 0-88275-955-8.







![{\displaystyle \phi ={\frac {C_{b}V_{b}}{C_{a}V_{a}}}={\frac {\alpha _{{\ce {A^-}}}-{\frac {{\ce {[H^+] - [OH^-]}}}{C_{a}}}}{1+{\frac {{\ce {[H^+] - [OH^-]}}}{C_{b}}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/18165b80d0eb0410df742c276c33e2c3ba309456)
![{\displaystyle \alpha _{A^{-}}={\frac {K_{a}}{[{\ce {H}}^{+}]+K_{a}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1f74f8a627346086e1008feb02618c57a8bb1fbf)

