Acyloin

In chemistry acyloins are a class of organic compounds which all possess the secondary α-hydroxy ketone functional group. Thus, they all contain a hydroxyl group placed on the α-position of a carbonyl group. The named acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups.[1]
Synthesis of acyloins
Classic organic reactions exist for the synthesis of acyloins.
- The acyloin condensation is a reductive coupling of esters
- The benzoin condensation is condensation reaction between aldehydes catalyzed by a nucleophile
- Oxidation of carbonyls is possible with molecular oxygen but not selective
- Better alternative is oxidation of corresponding silyl enol ethers with mCPBA in the Rubottom oxidation
- MoOPH oxidation of carbonyls is a system with molybdenum peroxide, pyridine and hexamethylphosphoramide.
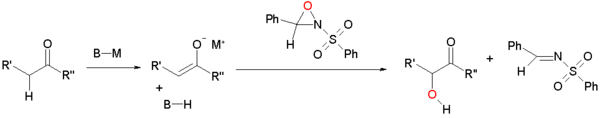
Enolate oxidation by sulfonyloxaziridines
Enolates can be oxidized by sulfonyloxaziridines.[2][3] The enolate reacts by nucleophilic displacement at the electron deficient oxygen of the oxaziridine ring.
This reaction type is extended to asymmetric synthesis by the use of chiral oxaziridines derived from camphor (camphorsulfonyl oxaziridine). Each isomer gives exclusive access to one of the two possible enantiomers. This modification is applied in the Holton taxol total synthesis.
In the enolate oxidation of the cyclopentaenone below[4] with either camphor enantiomer, the trans isomer is obtained because access for the hydroxyl group in the cis position is limited. The use of the standard oxaziridine did not result in an acyloin.
Reactions of acyloins
- Reduction of acyloins give diols.
- Oxidation of acyloins give diones.
- Some acyloins rearrange with positions swapped under the influence of base in the Lobry–de Bruyn–van Ekenstein transformation
- A similar reaction is the so-called Voigt amination[5] where an acyloin reacts with a primary amine and phosphorus pentoxide to an α-keto amine:[6]
- Indole synthesis,[7] compare Bischler–Möhlau
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "acyloins". doi:10.1351/goldbook.A00126
- ^ Davis, Franklin A.; Vishwakarma, Lal C.; Billmers, Joanne G.; Finn, John (1984). "Synthesis of α-hydroxycarbonyl compounds (acyloins): direct oxidation of enolates using 2-sulfonyloxaziridines". J. Org. Chem. 49 (17): 3241–3243. doi:10.1021/jo00191a048.
- ^ Davis, F. A.; Haque, M. S.; Ulatowski, T. G.; Towson, J. C. (1986). "Asymmetric oxidation of ester and amide enolates using new (camphorylsulfonyl)oxaziridines". J. Org. Chem. 51: 2402. doi:10.1021/jo00362a053.
- ^ a b Hughes, Chambers C.; Miller, Aubry K.; Trauner, Dirk (2005). "An Electrochemical Approach to the Guanacastepenes" (PDF). Org. Lett. 7 (16): 3425–3428. doi:10.1021/ol047387l. Archived from the original (PDF) on 4 September 2006.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ von Meyer, E.; Voigt, Karl (1886). "Ueber die Einwirkung von primären aromatischen Aminen auf Benzoïn" [On the effect of primary aromatic amines on benzoin]. J. Prakt. Chem. (in German). 34 (1): 1–27. doi:10.1002/prac.18860340101.
- ^ Lawrence, Stephen A. (2004). Amines: Synthesis, Properties and Applications. Cambridge University Press. ISBN 0-521-78284-8.
- ^ Roth, Lepke (1972). "Synthese von Indol- und Carbazol-Derivaten aus α-Hydroxyketonen und aromatischen Aminen" [Synthesis of indole and carbazole derivatives from α-hydroxyketones and aromatic amines]. Arch. Pharm. (in German). 305 (3): 159–171. doi:10.1002/ardp.19723050302.


![Enolate oxidation example[4]](http://upload.wikimedia.org/wikipedia/commons/thumb/0/02/Acyloin_example_Hughes.png/500px-Acyloin_example_Hughes.png)
