Ammonium dichromate

| ||||
| ||||

| ||||
| Names | ||||
|---|---|---|---|---|
| IUPAC name
Ammonium dichromate
| ||||
| Other names
Ammonium pyrochromate
| ||||
| Identifiers | ||||
3D model (JSmol)
|
||||
| ChemSpider | ||||
| ECHA InfoCard | 100.029.221 | |||
| RTECS number |
| |||
| UN number | 1439 | |||
CompTox Dashboard (EPA)
|
||||
| ||||
| ||||
| Properties | ||||
| (NH4)2Cr2O7 | ||||
| Molar mass | 252.07 g/mol | |||
| Appearance | Orange-red crystals | |||
| Density | 2.115 g/cm3 | |||
| Melting point | 180 °C (decomp.) | |||
| 18.2 g/100 mL (0 °C) 26.67 g/100 mL (20 °C) 156 g/100 mL (100 °C) | ||||
| Solubility | insoluble in acetone soluble in alcohol | |||
| Hazards | ||||
| GHS labelling: | ||||
| class="wikitable collapsible" style="min-width: 50em;" | ||||
| Pictogram | Code | Symbol description | Image link | |
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
- {{H-phrases}}
- {{P-phrases}}
- Category:GHS templates
| Pictogram | Code | Symbol description | Image link | |
|---|---|---|---|---|
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
- {{H-phrases}}
- {{P-phrases}}
- Category:GHS templates
| Pictogram | Code | Symbol description | Image link | |
|---|---|---|---|---|
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
- {{H-phrases}}
- {{P-phrases}}
- Category:GHS templates
| Pictogram | Code | Symbol description | Image link | |
|---|---|---|---|---|
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
- {{H-phrases}}
- {{P-phrases}}
- Category:GHS templates
| Pictogram | Code | Symbol description | Image link | |
|---|---|---|---|---|
 |
GHS01 | {{GHS exploding bomb}} | Image:GHS-pictogram-explos.svg | Explosive |
 |
GHS02 | {{GHS flame}} | Image:GHS-pictogram-flamme.svg | |
 |
GHS03 | {{GHS flame over circle}} | Image:GHS-pictogram-rondflam.svg | |
 |
GHS04 | {{GHS gas cylinder}} | Image:GHS-pictogram-bottle.svg | |
 |
GHS05 | {{GHS corrosion}} | Image:GHS-pictogram-acid.svg | Corrosive |
 |
GHS06 | {{GHS skull and crossbones}} | Image:GHS-pictogram-skull.svg | Accute Toxic |
 |
GHS07 | {{GHS exclamation mark}} | Image:GHS-pictogram-exclam.svg | Irritant |
 |
GHS08 | {{GHS health hazard}} | Image:GHS-pictogram-silhouette.svg | Health Hazard |
 |
GHS09 | {{GHS environment}} | Image:GHS-pictogram-pollu.svg | Environment |
See also
|-
|-
| style="padding-left:1em;" |
| H272, H301, H312, H314, H317, H330, H334, H340, H350, H360, H372, H410[2]
|-
|-
| style="padding-left:1em;" |
| P201, P220, P260, P273, P280, P284[2]
|- | NFPA 704 (fire diamond)
|
|- ! colspan=2 style="background: #f8eaba; text-align: center;" |Related compounds
|-
|
| Potassium dichromate
Sodium dichromate
|-
| colspan=2 style="text-align:left; background:#f8eaba; border:1px solid #a2a9b1;" |
|-
|}
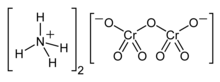
Ammonium dichromate is the inorganic compound with the formula (NH4)2Cr2O7. In this compound, as in all chromates and dichromates, chromium is in a +6 oxidation state, commonly known as hexavalent chromium. It is a salt consisting of ammonium ions and dichromate ions.
Ammonium dichromate is sometimes known as Vesuvian Fire, because of its use in demonstrations of tabletop "volcanoes".[3] It has been used in pyrotechnics and in the early days of photography.
Properties
At room temperature and pressure, the compound exists as orange, acidic crystals soluble in water and alcohol. It is formed by the action of chromic acid on ammonium hydroxide with subsequent crystallisation.[4]
The (NH4)2Cr2O7 crystal (C2/c, z=4) contains a single type of ammonium ion, at sites of symmetry C1(2,3). Each NH4+ centre is surrounded irregularly by eight oxygen atoms at N---O distances ranging from ca. 2.83 to ca. 3.17 Å, typical of hydrogen bonds.[5]
Uses
It has been used in pyrotechnics and in the early days of photography as well as in lithography, as a source of pure nitrogen in the laboratory, and as a catalyst.[6] It is also used as a mordant for dyeing pigments, in the manufacturing of alizarin, chrome alum, leather tanning and oil purification.[4]
Photosensitive films containing PVA, ammonium dichromate, and a phosphor are spin-coated as aqueous slurries in the production of the phosphor raster of television screens and other devices. The ammonium dichromate acts as the photoactive site.[7]
Reactions
Tabletop volcanoes and thermal decomposition
The volcano demonstration involves igniting a pile of the salt, which initiates the following exothermic conversion:[8]
- (NH
4)
2Cr
2O
7 (s) → Cr
2O
3 (s) + N
2 (g) + 4 H
2O (g) (ΔH=−429.1 ± 3 kcal/mol)
Like the well-known explosive ammonium nitrate, ammonium dichromate contains both an oxidizer (dichromate) and a reducer (ammonium), making it thermodynamically unstable.[9][10] Its decomposition reaction proceeds to completion once initiated, producing voluminous dark green powdered chromium(III) oxide. Not all of the ammonium dichromate decomposes in this reaction. When the green powder is brought into water a yellow/orange solution is obtained from left over ammonium dichromate.
Observations obtained using relatively high magnification microscopy during a kinetic study of the thermal decomposition of ammonium dichromate provided evidence that salt breakdown proceeds with the intervention of an intermediate liquid phase rather than a solid phase. The characteristic darkening of (NH
4)
2Cr
2O
7 crystals as a consequence of the onset of decomposition can be ascribed to the dissociative loss of ammonia accompanied by progressive anion condensation to Cr
3O2−
10, Cr
4O2−
13, etc., ultimately yielding CrO
3. The CrO
3 has been identified as a possible molten intermediate participating in (NH
4)
2Cr
2O
7 decomposition.[11]
Oxidation reactions
Ammonium dichromate is a strong oxidising agent and reacts, often violently, with any reducing agent. The stronger the reducing agent, the more violent the reaction.[9] It has also been used to promote the oxidation of alcohols and thiols. Ammonium dichromate, in the presence of Mg(HSO4)2 and wet SiO2 can act as a very efficient reagent for the oxidative coupling of thiols under solvent free conditions. The reactions produces reasonably good yields under relatively mild conditions.[12] The compound is also used in the oxidation of aliphatic alcohols to their corresponding aldehydes and ketones in ZrCl4/wet SiO2 in solvent free conditions, again with relatively high yields.[13][14]
Safety
Ammonium dichromate, like all chromium(VI) compounds, is highly toxic and a suspected carcinogen.[15] It is also a strong irritant.
Incidents
In sealed containers, ammonium dichromate is likely to explode if heated.[9] On January 19, 1986, The New York Times reported that two workers had been killed and 14 others injured at Diamond Shamrock Chemicals, Ashtabula, Ohio when 2000 lbs of ammonium dichromate exploded as they were being dried in a heater.[16]
References
- ^ a b c d e "Globally Harmonized System of Classification and Labelling of Chemicals" (pdf). 2021. Annex 3: Codification of Statements and Pictograms (pp 268–385).
- ^ a b c Template:SigmaLink
- ^ "Ammonium Dichromate Volcano". Chemistry Comes Alive!. J. Chem. Ed.
- ^ a b Richard J. Lewis Hawley's Condensed Chemical Dictionary. Wiley & Sons, Inc: New York, 2007 ISBN 978-0-471-76865-4
- ^ Keresztury, G and Knop, O. (1982). "Infrared spectra of the ammonium ion in crystals. Part XII. Low-temperature transitions in ammonium dichromate, (NH4)2Cr2O7". Can. J. Chem.: 1972–1976.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ Havard, J.M., Shim, S.Y., and Fréchet, J.M. (1999). "Design of Photoresists with Reduced Environmental Impact. 1. Water-Soluble Resists Based on Photo-Cross-Linking of Poly(vinyl alcohol)". Chem. Mater. 11: 719–725. doi:10.1021/cm980603y.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Neugebauer, C. A and Margrave, J.L. (1957). "The Heat Formation of Ammonium Dichromate". J. Phys. Chem. 61 (10): 1429–1430. doi:10.1021/j150556a040.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Young, A.J. (2005). "CLIP, Chemical Laboratory Information Profile: Ammonium Dichromate". J. Chem. Educ. 82: 1617. doi:10.1021/ed082p1617.
- ^ G.A.P. Dalgaard, A.C. Hazell and R.G. Hazell (1974). ""The Crystal Structure of Ammonium Dichromate, (NH4)2Cr2O7". Acta Chemica Scandinavica. A28: 541–545. doi:10.3891/acta.chem.scand.28a-0541.
- ^ Galwey, A.K., PÖppl, L, and Rajam, S. (1983). "A Melt Mechanism for the Thermal Decomposition of Ammonium Dichromate". J. Chem. Soc., Faraday Trans. 1. 79: 2143–2151. doi:10.1039/f19837902143.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shirini, F.; et al. (2003). "Solvent free oxidation of thiols by (NH4)2Cr2O7 in the presence of Mg(HSO4)2 and wet SiO2". J. Chem. Research (S). 2003: 28–29. doi:10.3184/030823403103172823.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Shirini, F.; et al. (2001). "ZrCl4/wet SiO2 promoted oxidation of alcohols by (NH4)2Cr2O7 in solution and solvent free condition". J. Chem. Research (S). 2001: 467–477. doi:10.3184/030823401103168541.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ F. Shirini, M. A. Zolfigol,† and M. Khaleghi (2003). "Oxidation of Alcohols Using (NH4)2Cr2O7 in the Presence of Silica Chloride/Wet SiO2 in Solution and under Solvent Free Conditions". Bull. Korean Chem. Soc. 24 (7): 1021–1022. doi:10.5012/bkcs.2003.24.7.1021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Volkovich, V.A. and Griffiths, T.R. (2000). "Catalytic Oxidation of Ammonia: A Sparkling Experiment". J. Chem. Ed. 77 (2): 177. doi:10.1021/ed077p177.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Diamond, S. The New York Times, 1986, p 22.

