Bartoli indole synthesis
The Bartoli indole synthesis (also called the Bartoli reaction) is the chemical reaction of ortho-substituted nitroarenes with vinyl grignard reagents to form substituted indoles.[1][2][3][4]
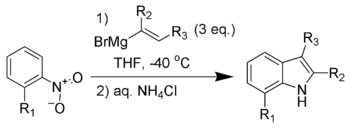
The reaction is unsuccessful without substitution ortho to the nitro group. Three equivalents of the vinyl grignard reagent are also necessary for good yields.
This method has become one of the shortest and most flexible routes to 7-substituted indoles.[5][6] The Leimgruber-Batcho indole synthesis gives similar flexibility and regiospecificity to indole derivatives. One advantage of the Bartoli indole synthesis is the ability to produce indoles substituted on both the carbocyclic ring and the pyrrole ring, which is difficult to do with the Leimgruber-Batcho indole synthesis.
Reaction mechanism
The reaction mechanism[7] of the Bartoli indole synthesis is illustrated below using o-nitrotoluene (1) and propenyl grignard (2) to form 3,7-dimethylindole (13).

The mechanism begins by the addition of the grignard reagent (2) onto the nitroarene (1) to form intermediate 3. Intermediate 3 spontaneously decomposes to form a nitrosoarene (4) and a magnesium salt (5). (Upon reaction workup, the magnesium salt will liberate a carbonyl compound (6).) Reaction of the nitrosoarene (4) with a second equivalent of the grignard reagent (2) forms intermediate 7. The steric bulk of the ortho group causes a [3,3]-sigmatropic rearrangement forming the intermediate 8. Cyclization and tautomerization give intermediate 10, which will react with a third equivalent of the grignard reagent (2) to give a dimagnesium indole salt (12). Reaction workup eliminates water and gives the final desired indole (13).
Therefore, three equivalents of the grignard reagent are necessary, as one equivalent becomes a carbonyl compound (6), one equivalent gets protonated forming an alkene (11), and one equivalent gets incorporated into the indole ring.
The nitroso intermediate (4) has been isolated from the reaction. Additionally, reaction of the nitroso intermediate (4) with two equivalents of the grignard reagent produces the expected indole.
Variations
Dobbs modification
Adrian Dobbs greatly enhanced the scope of the Bartoli indole synthesis by using an ortho-bromine as a directing group, which is subsequently removed by AIBN and tributyltin hydride.[8]
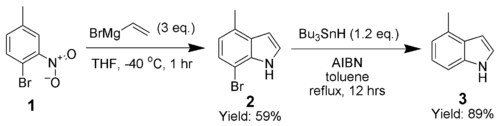
The synthesis of 4-methylindole (3) highlights the ability of this technique to produce highly substituted indoles.
References
- ^ Bartoli, G; Palmieri, G; Bosco, M; Dalpozzo, R (1989). "The reaction of vinyl grignard reagents with 2-substituted nitroarenes: A new approach to the synthesis of 7-substituted indoles". Tetrahedron Lett. 30: 2129–2132. doi:10.1016/S0040-4039(01)93730-X.)
- ^ Bartoli, G.; Bosco, M.; Dalpozzo, R.; Palmieri, G.; Marcantoni, E. (1991). "Reactivity of nitro- and nitroso-arenes with vinyl grignard reagents: synthesis of 2-(trimethylsilyl)indoles". J. Chem. Soc., Perkin Trans. 1. 1: 2757–2761. doi:10.1039/p19910002757.
- ^ Dobbs, A. P.; Voyle, M.; Whitall, N. (1999). "Synthesis of Novel Indole Derivatives: Variations in the Bartoli Reaction". Synlett. 1999: 1594–1596. doi:10.1055/s-1999-2900.
- ^ Dalpozzo, R.; Bartoli, G. (2005). "Bartoli Indole Synthesis" (PDF). Curr. Org. Chem. 9: 163–178. doi:10.2174/1385272053369204.
- ^ Dobson, D.; Todd, A.; Gilmore, J. (1991). "The Synthesis of 7-Alkoxyindoles". Synth. Commun. 21: 611–617. doi:10.1080/00397919108020827.
- ^ Dobson, D. R.; Gilmore, J.; Long, D. A. (1992). "Synthesis of 7-Formylindole Using the Bartoli Indole Methodology". Synlett. 1992: 79–80. doi:10.1055/s-1992-21273.
- ^ Bosco, M.; Dalpozzo, R.; Bartoli, G.; Palmieri, G.; Petrini, M. J. Chem. Soc. Perkin Trans. 2 1991, 657-663.
- ^ Dobbs, A (2001). "Total Synthesis of Indoles from Tricholoma Species via Bartoli/Heteroaryl Radical Methodologies". J. Org. Chem. 66: 638–641. doi:10.1021/jo0057396.
