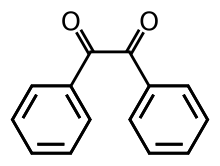
Benzil
| |

| |
| |
| Names | |
|---|---|
| IUPAC name
1,2-diphenylethane-1,2-dione
| |
| Other names
dibenzoyl
bibenzoyl diphenylglyoxal | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.689 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H10O2 | |
| Molar mass | 210.232 g·mol−1 |
| Appearance | yellow crystals or powder |
| Density | 1.23 g/cm3, solid (1.255 g/cm3, x-ray) |
| Melting point | 94-96 °C, 262.14–262.63 °F, 401-402 K |
| Boiling point | 346–348 °C, 654.8–658.4 °F, 619–621 K |
| insoluble | |
| Solubility in benzene | soluble |
| Structure | |
| P31,221[1] | |
| 3.8 D[2] | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzil (systematically known as 1,2-diphenylethane-1,2-dione) is the organic compound with the formula (C6H5CO)2, generally abbreviated (PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoinitiator in polymer chemistry.[3]
Structure
The compound's most noteworthy structural feature is the long carbon-carbon bond of 1.54 Å, which indicates the absence of pi-bonding between the two carbonyl centers. The PhCO centers are planar, but the pair of benzoyl groups are twisted with respect to the other with a dihedral angle of 117°.[4] In less hindered analogues (glyoxal, biacetyl, oxalic acid derivatives), the (RCO)2 group adopts a planar, anti-conformation.
Applications
Most benzil is consumed for use in the free-radical curing of polymer networks. Ultraviolet radiation decomposes benzil, generating free-radical species within the material, promoting the formation of cross-links. Recently, benzil has been demonstrated to be a potent inhibitor of human carboxylesterases, enzymes involved in the hydrolysis of carboxylesters and many clinically used drugs. [5]
Reactions
Benzil is a standard building block in organic synthesis. It condenses with amines to give diketimines ligands. A classic organic reaction of benzil is the benzilic acid rearrangement, in which base catalyses the conversion of benzil to benzilic acid. This reactivity is exploited in the preparation of the drug phenytoin. Benzil also reacts with 1,3-diphenylacetone in an aldol condensation to give tetraphenylcyclopentadienone.
Preparation
Benzil is prepared from benzoin, which in turn is easily obtained via the benzoin condensation from benzaldehyde.[6]
- PhC(O)CH(OH)Ph + 2 Cu2+ → PhC(O)C(O)Ph + 2 H+ + 2 Cu+
References
- ^ Acta Cryst. B43 398 (1987)
- ^ Spectrochim. Acta A60 (8-9) 1805 (2004)
- ^ Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Weinheim. doi:10.1002/14356007.a15_077
- ^ Quang. Shen, Kolbjoern. Hagen "Gas-phase molecular structure and conformation of benzil as determined by electron diffraction" J. Phys. Chem., 1987, 91 (6), pp 1357–1360. doi:10.1021/j100290a017.
- ^ Wadkins. R. M. et al "Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases. J. Med. Chem., 2005 48 pp 2906-15.
- ^ Clarke, H. T.; Dreger.E. E. (1941). "Benzil". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 1, p. 87.

