Cell migration
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (May 2009) |
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic development, wound healing and immune responses all require the orchestrated movement of cells in particular directions to specific locations. Errors during this process have serious consequences, including mental retardation, vascular disease, tumor formation and metastasis. An understanding of the mechanism by which cells migrate may lead to the development of novel therapeutic strategies for controlling, for example, invasive tumour cells. Cells often migrate in response to, and towards, specific external signals, a process called chemotaxis.
Studying cell migration
The migration of single mammalian cells is usually viewed in the microscope as the cells move randomly on a glass slide. As the actual movement is very slow — usually a few micrometers/minute — time-lapse films are taken so that a speeded up movie can be viewed.[1] This shows that, although the shape of a moving cell varies considerably, its leading front has a characteristic behaviour. This region of the cell is highly active, sometimes spreading forwards quickly, sometimes retracting, sometimes ruffling or bubbling. It is generally accepted that the leading front is the main motor which pulls the cell forward.
Common features
There is still great uncertainty of how cell migration really works. However, because the locomotion of all mammalian cells (except sperm) has several common features, the underlying processes are believed to be similar. The two main constant features are:
- the behaviour of the leading front and
- the observation that any debris on the dorsal surface of the cell moves backwards on the cell’s surface towards its trailing end. The latter feature is most easily observed when aggregates of a surface molecule are cross-linked with a fluorescent antibody (see cap formation) or when small beads become artificially bound to the front of the cell.[2]
Besides mammalian cells, many other eukaryotic cells appear to move in a similar way. One of the most valuable model creatures for studying locomotion and chemotaxis is the amoeba Dictyostelium discoideum because they move more quickly than most mammalian cells grown in the lab and they chemotax towards cyclic AMP. In addition, they have a haploid genome which assists understanding the role of a particular gene product in movement.
Molecular processes at the front
There are two main theories for how the cell advances its front edge: the cytoskeletal model and membrane flow model. It is possible that both underlying processes contribute to cell extension.
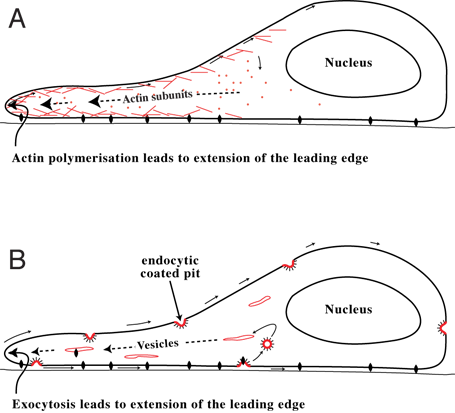
Cytoskeletal model (A)
Experimentally it is found that the cell's front is a site of rapid actin polymerisation: soluble actin monomers polymerise there to form filaments.[3] This has led to the view that it is the formation of these actin filaments which pushes the leading front forward and is the main motile force for advancing the cell’s front.[4][5] In addition, cytoskeletal elements are able to interact extensively and intimately with a cell's plasma membrane.[6]
Membrane flow model (B)
Studies have also shown that the front is the site at which membrane is returned to the cell surface from internal membrane pools at the end of the endocytic cycle.[7] This has led to the view that extension of the leading edge occurs primarily by addition of membrane at the front of the cell. If so, the actin filaments which form at the front might stabilize the added membrane so that a structured extension, or lamella, is formed rather than the cell blowing bubbles (or "blebs") at its front.[8] For a cell to move, it is necessary to bring a fresh supply of feet — those molecules, called integrins, which attach a cell to the surface on which it is crawling — to the front. It is likely that these feet are endocytosed towards the rear of the cell and brought to the cell's front by exocytosis, to be reused to form new attachments to the substrate.
The nucleus and rear
Given that a cell’s front advances, what about the rest of the cell? Is it simply dragged forward, like a sack? We do not know, but there are suggestions that the nucleus and perhaps other large structures inside the cell may also be pulled forward by actin filaments. In addition, it may be that the rear of the cell actively contracts, as it is here that, in some cells, the major contractile protein myosin is found.
Mutants
Insight into how complex biological processes work can often be gleaned from a study of mutations. In the case of the intracellular mechanisms underlying cell movement, this has been largely unsuccessful. Thus, although many mutants are known in Drosophila which affect migratory processes, these tend to fall into two groups: transcription factors (such as slow border cells (slbo) which affects the migration of the border cells) or key regulator proteins (such as C-Jun N-terminal kinases (JNK) which controls dorsal closure). These, however, tell us little about how cells actually move.
Another major source of mutants is the haploid amoeba Dictyostelium. Many single copy genes associated with cytoskeletal function have been deleted: these mutants usually have only a weak phenotype, suggesting either that these genes are not required for locomotion or that there are multiple mechanisms by which cells can move.[9] However, temperature-sensitive mutants in the genes for N-ethylmaleimide sensitive fusion protein (NSF) and Sec1 rapidly block cell migration[10][11] indicating that the NSF protein and Sec1p are both required for some aspects of cell movement. NSF is known to function in intracellular membrane fusion;[10] Sec1p in yeast is required for polarised exocytosis.[11]
Polarity in migrating cells
Migrating cells clearly have a polarity: a front and a back. Without it, they would move in all directions at once, or spread. How this arrow is formulated at a molecular level inside a cell is unknown. In a cell which is meandering in a random way, the front can easily give way to become passive as some other region, or regions, of the cell form(s) a new front. In chemotaxing cells, the stability of the front appears enhanced as the cell advances towards a higher concentration of the stimulating chemical. This polarity is reflected at a molecular level by a restriction of certain molecules to particular regions of the inner cell surface: thus the phospholipid PIP3 and activated Rac and CDC42 are found at the front of the cell, whereas Rho GTPase and PTEN are found towards the rear.[12][13]
It is believed that microtubules and filamentous actin are important for establishing and maintaining a cell’s polarity. Thus, drugs which destroy microtubules disrupt the polarity of many cells: if the cell is attached to a substratum, they often become round and flat. Drugs which destroy actin filaments have multiple and complex effects, reflecting the wide role that these filaments play in many cell processes. It may be that, as part of the locomotory process, membrane vesicles are transported along these filaments to the cell’s front. In chemotaxing cells, the increased persistence of migration towards the target may result from an increased stability of the arrangement of the filamentous structures inside the cell and which determine its polarity. In turn, these filamentous structures may be arranged inside the cell according to how molecules like PIP3 and PTEN are arranged on the inner cell surface. And where these are located appears in turn to be determined by the chemoattractant signals as these impinge on specific receptors on the cell’s outer surface.
See also
References
- ^ http://www.microscopyu.com/moviegallery/livecellimaging/mdbk/index.html/ movie of MDBK cells moving over a glass slide
- ^ Abercrombie, M; Heaysman, JE; Pegrum, SM (1970). "The locomotion of fibroblasts in culture III. Movements of particles on the dorsal surface of the leading lamella". Experimental Cell Research. 62 (2): 389–98. doi:10.1016/0014-4827(70)90570-7. PMID 5531377.
- ^ Wang, Y. L. (1985). "Exchange of actin subunits at the leading edge of living fibroblasts: possible role of treadmilling". The Journal of Cell Biology. 101 (2): 597–602. doi:10.1083/jcb.101.2.597. PMC 2113673. PMID 4040521.
- ^ Mitchison, T; Cramer, LP (1996). "Actin-Based Cell Motility and Cell Locomotion". Cell. 84 (3): 371–9. doi:10.1016/S0092-8674(00)81281-7. PMID 8608590.
- ^ Pollard, Thomas D; Borisy, Gary G (2003). "Cellular Motility Driven by Assembly and Disassembly of Actin Filaments". Cell. 112 (4): 453–65. doi:10.1016/S0092-8674(03)00120-X. PMID 12600310.
- ^ Doherty, Gary J.; McMahon, Harvey T. (2008). "Mediation, Modulation, and Consequences of Membrane-Cytoskeleton Interactions". Annual Review of Biophysics. 37: 65–95. doi:10.1146/annurev.biophys.37.032807.125912. PMID 18573073.
- ^ Bretscher, M. S. (1983). "Distribution of Receptors for Transferrin and Low Density Lipoprotein on the Surface of Giant HeLa Cells". Proceedings of the National Academy of Sciences. 80: 454–8. doi:10.1073/pnas.80.2.454.
- ^ Bretscher, M (1996). "Getting Membrane Flow and the Cytoskeleton to Cooperate in Moving Cells". Cell. 87 (4): 601–6. doi:10.1016/S0092-8674(00)81380-X. PMID 8929529.
- ^ Noegel, AA; Schleicher, M (2000). "The actin cytoskeleton of Dictyostelium: a story told by mutants". Journal of cell science. 113 (Pt 5): 759–66. PMID 10671366.
- ^ a b Thompson, CR; Bretscher, MS (2002). "Cell polarity and locomotion, as well as endocytosis, depend on NSF". Development. 129 (18): 4185–92. PMID 12183371.
- ^ a b Bretscher, Mark S.; Clotworthy, Margaret; Zhang, Shuguang (2007). "Using Single loxP Sites to Enhance Homologous Recombination: ts Mutants in Sec1 of Dictyostelium discoideum". PLoS ONE. 2 (1): e724. doi:10.1371/journal.pone.0000724. PMC 1933600. PMID 17684569.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Parent, C. A.; Devreotes, PN (1999). "A Cell's Sense of Direction". Science. 284 (5415): 765–70. doi:10.1126/science.284.5415.765. PMID 10221901.
- ^ Ridley, A. J.; Schwartz, MA; Burridge, K; Firtel, RA; Ginsberg, MH; Borisy, G; Parsons, JT; Horwitz, AR (2003). "Cell Migration: Integrating Signals from Front to Back". Science. 302 (5651): 1704–9. doi:10.1126/science.1092053. PMID 14657486.
External links
- Cell Migration Gateway The Cell Migration Gateway is a comprehensive and regularly updated resource on cell migration
- http://cellix.imba.oeaw.ac.at/ Video tour of cell motility
