Chlorophyll c
You can help expand this article with text translated from the corresponding article in Serbo-Croatian. (August 2014) Click [show] for important translation instructions.
|
Chlorophyll c is a form of chlorophyll found in certain marine algae, including the photosynthetic Chromista (e.g. diatoms, brown algae) and dinoflagellates.[1][2][3]
It has a blue-greenish color and is an accessory pigment, particularly significant in its absorption of light in the 447-452 nm region, [3] Like chlorophyll a and chlorophyll b, it helps the organism gather light and passes a quanta of excitation energy through the light harvesting antennae to the photosynthetic reaction centre. Chlorophyll c is unusual because it has a porphyrin ring structure and does not have an isoprenoid tail or a reduced ring D, features typical of the other chlorophylls commonly found in algae and plants.[2]
Chlorophyll c can be further divided into chlorophyll c1, chlorophyll c2[3] and chlorophyll c3,[4] plus at least 8 other newly found subtypes.[5]
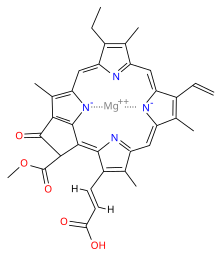
Chlorophyll c1
| |
| |
| Names | |
|---|---|
| IUPAC name
[(2E)-3-[14-Ethyl-21-(methoxycarbonyl)-4,8,13,18-tetramethyl-20-oxo-9-vinyl-3,4-didehydro-3-phorbinyl-κ2N23,N25]acrylato(2-)]magnesium
| |
| Identifiers | |
| ChemSpider | |
PubChem CID
|
|
| Properties | |
| C35H30MgN4O5 | |
| Molar mass | 610.953 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorophyll c1 is a common form of chlorophyll c. It differs from chlorophyll c2 in its C8 group, having an ethyl group instead of vinyl group (C-C single bond instead of C=C double bond). Its absorption maxima are around 444, 577, 626 nm and 447, 579, 629 nm in diethyl ether and acetone respectively.[6]
Chlorophyll c2

| |

| |
| Names | |
|---|---|
| IUPAC name
[(2E)-3-[21-(Methoxycarbonyl)-4,8,13,18-tetramethyl-20-oxo-9,14-divinyl-3,4-didehydro-3-phorbinyl-κ2N23,N25]acrylato(2-)]magnesium
| |
| Identifiers | |
| ChemSpider | |
PubChem CID
|
|
| Properties | |
| C35H28MgN4O5 | |
| Molar mass | 608.937 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorophyll c2 is the most common form of chlorophyll c.[7] Its absorption maxima are around 447, 580, 627 nm and 450, 581, 629 nm in diethyl ether and acetone respectively.[6]
Chlorophyll c3
| Identifiers | |
|---|---|
| Properties | |
| C36H28MgN4O7 | |
| Molar mass | 652.946 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorophyll c3 is a form of chlorophyll c found in microalga Emiliania huxleyi, identified in 1989.[4] Its absorption maxima are around 452, 585, 625 nm and 452, 585, 627 nm in diethyl ether and acetone respectively.[6]
References
- ^ Speer, B.R. "Photosynthetic Pigments". Retrieved 2 August 2014.
- ^ a b Blankenship, Robert E. (February 2002). Molecular Mechanisms of Photosynthesis. Wiley-Blackwell.
- ^ a b c Dougherty, Ralph C.; Strain, H. H.; Svee, W. A.; Uphaus, R. A.; Katz, J. J. (May 1970). "The Structure, Properties, and Distribution of Chlorophyll c". J. Am. Chem. Soc. 92 (9): 2826–2833. doi:10.1021/ja00712a037.
- ^ a b Fookes, Christopher J. R.; Jeffrey, S. W. (1989). "The structure of chlorophyll c3, a novel marine photosynthetic pigment". J. Chem. Soc., Chem. Commun. (23): 1827–1828.
- ^ Zapata, Manuel; Garrido, José L.; Jeffrey, Shirley W. (2006). "Chlorophyll c Pigments: Current Status". Chlorophylls and Bacteriochlorophylls: Advances in Photosynthesis and Respiration. 25: 39–53. doi:10.1007/1-4020-4516-6_3.
- ^ a b c Fawley, Marvin W. (1989). "A New Form of Chlorophyll c Involved in Light-Harvesting". Plant Physiol. 91 (2): 727–732. doi:10.1104/pp.91.2.727. PMC 1062062. PMID 16667093.
- ^ Jeffrey, S. W. (28 June 2014). "The Occurrence of Chlorophyll c1 and c2 in Algae". Journal of Phycology. 12 (3): 349–354. doi:10.1111/j.1529-8817.1976.tb02855.x.
