Coupling reaction
A coupling reaction in organic chemistry is a general term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst. In one important reaction type a main group organometallic compound of the type RM (R = organic fragment, M = main group centre) reacts with an organic halide of the type R'X with formation of a new carbon-carbon bond in the product R-R' [1][2]
Richard F. Heck Ei-ichi Negishi and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium catalyzed cross coupling reactions.[3][4]
Broadly speaking, two types of coupling reactions are recognized:
- heterocouplings couple two different partners, for example the Heck reaction of an Alkene (RC=CH) and an Alkyl halide (R'-X) to give a substituted Alkene (RC=CR').
- homocouplings couple two identical partners, for example, the Glaser coupling of two Acetylideo(RC≡CH) to form a dialkyne (RC≡C-C≡CR).
Mechanism
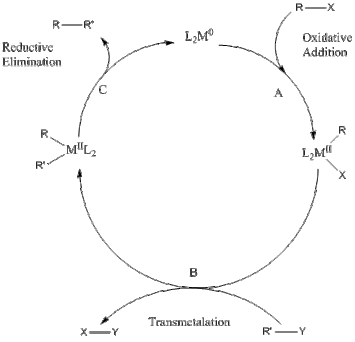
The reaction mechanism generally begins with the oxidative addition of an organic halide to the catalyst. Subsequently, the second partner undergoes transmetallation, which places both coupling partners on the same metal center while eliminating the functional groups. The final step is reductive elimination of the two coupling fragments to regenerate the catalyst and give the organic product. Unsaturated organic groups couple more easily in part because they add readily. The intermediates are also less prone to beta-hydride elimination.[5]
In one computational study, unsaturated organic groups were shown to undergo much easier coupling reaction on the metal center.[6] The rates for reductive elimination followed the following order: vinyl-vinyl > phenyl-phenyl > alkynyl-alkynyl > alkyl-alkyl. The activation barriers and the reaction energies for unsymmetrical R-R′ couplings were found to be close to the averages of the corresponding values of the symmetrical R-R and R′-R′ coupling reactions; for example: vinyl-vinyl > vinyl-alkyl > alkyl-alkyl. Another mechanistic approach proposes that specifically in aqueous solutions, coupling actually occurs via a radical mechanism rather than a metal-assisted one.[7] Most of the coupling reaction's mechanisms slightly vary from this generalized form.
Catalysts
The most common catalyst is palladium, but an increasing number of reactions use nickel. Other catalysts include copper, platinum, iron, cobalt, and amines.
Palladium is robust catalyst and is frequently used due to high functional group tolerance, and low sensitivity of organopalladium compounds towards water and air. However, palladium is a quite rare and costly noble metal. Additionally palladium catalysts are notoriously difficult to remove. Purification typically involves extensive column chromatography, recrystallization, metal scavengers, distillation, or extraction to name a few techniques. Most methods typically do not completely remove the catalyst. This typically causes issues for the pharmaceutical industry which faces extensive regulation regarding heavy metals. Many pharmaceutical chemists attempt to use coupling reactions early in production to minimize metal traces in the product.[8]
Nickel catalysts, while less robust than palladium ones, are cheaper, easier to remove, and less toxic. Nickel catalysts frequently require energetic substrates or co-catalysts such as Photoredox catalysts. Currently, many research groups are trying to create heterogeneous reusable catalysts to minimize cost and reduce purification needs.
Most catalysts use bulky L type ligands such as triphenylphosphine,
In depth-reviews have been written for example on cobalt,[9] palladium [10][11][12][13][14] and nickel [15] mediated reactions and on applications [16][17]
Leaving groups
The leaving group X in the organic partner is usually a halogen. Chloride is the most ideal group due to their low cost, but frequently have issues with reactivity. The main group metal in the organometallic partner usually is tin, zinc, silates or boron.
Operating conditions
While many coupling reactions involve reagents that are extremely susceptible to presence of water or oxygen, it is unreasonable to assume that all coupling reactions need to be performed with strict exclusion of water. It is possible to perform palladium-based coupling reactions in aqueous solutions using the water-soluble sulfonated phosphines made by the reaction of triphenyl phosphine with sulfuric acid. Another example of coupling in aqueous media, with the main reacting agent being trimolybdenum-alkylidyne clusters, is that of Bogoslavsky et al.[7] In general, the oxygen in the air is more able to disrupt coupling reactions, because many of these reactions occur via unsaturated metal complexes that do not have 18 valence electrons. For example, in nickel and palladium cross couplings, a zerovalent complex with two vacant sites (or labile ligands) reacts with the carbon halogen bond to form a metal halogen and a metal carbon bond. Such a zerovalent complex with labile ligands or empty coordination sites is normally very reactive toward oxygen.
Some catalysts might be easily poisoned by heterocycles under prolonged reaction at elevated temperature. To avoid this, chemists often use pressure reactors to accelerate reactions at high temperature and pressure. Q-Tube and microwave synthesizer are available safe pressure reactors.
Coupling types
Coupling reactions include (not exhaustive):
| Reaction | Year | Reactant A | Reactant B | Homo/Cross | Catalyst | Remark | ||
| Wurtz reaction | 1855 | R-X | sp3 | R-X | sp3 | homo | Na as reducing agent | |
| Glaser coupling | 1869 | RC≡CH | sp | RC≡CH | sp | homo | Cu | O2 as H-acceptor |
| Ullmann reaction | 1901 | Ar-X | sp2 | Ar-X | sp2 | homo | Cu | high temperatures |
| Gomberg-Bachmann reaction | 1924 | Ar-H | sp2 | Ar-N2X | sp2 | homo | requires base | |
| Cadiot-Chodkiewicz coupling | 1957 | RC≡CH | sp | RC≡CX | sp | cross | Cu | requires base |
| Pinacol coupling reaction | ||||||||
| Castro-Stephens coupling | 1963 | RC≡CH | sp | Ar-X | sp2 | cross | Cu | |
| Gilman reagent coupling | 1967 | R2CuLi | R-X | cross | ||||
| Cassar reaction | 1970 | Alkene | sp2 | R-X | sp3 | cross | Pd | requires base |
| Kumada coupling | 1972 | Ar-MgBr | sp2, sp3 | Ar-X | sp2 | cross | Pd or Ni or Fe | |
| Heck reaction | 1972 | alkene | sp2 | R-X | sp2 | cross | Pd or Ni | requires base |
| Sonogashira coupling | 1975 | RC≡CH | sp | R-X | sp3 sp2 | cross | Pd and Cu | requires base |
| Negishi coupling | 1977 | R-Zn-X | sp3, sp2, sp | R-X | sp3 sp2 | cross | Pd or Ni | |
| Stille cross coupling | 1978 | R-SnR3 | sp3, sp2, sp | R-X | sp3 sp2 | cross | Pd | |
| Suzuki reaction | 1979 | R-B(OR)2 | sp2 | R-X | sp3 sp2 | cross | Pd or Ni | requires base |
| Hiyama coupling | 1988 | R-SiR3 | sp2 | R-X | sp3 sp2 | cross | Pd | requires base |
| Buchwald-Hartwig reaction | 1994 | R2N-H | sp | R-X | sp2 | cross | Pd | N-C coupling, second generation free amine |
| Fukuyama coupling | 1998 | R-Zn-I | sp3 | RCO(SEt) | sp2 | cross | Pd or Ni[18] | |
| Liebeskind–Srogl coupling | 2000 | R-B(OR)2 | sp3, sp2 | RCO(SEt) Ar-SMe | sp2 | cross | Pd | requires CuTC |
| Coupling reaction overview. For references consult satellite pages | ||||||||
Miscellaneous reactions
In one study, an unusual coupling reaction was described in which an organomolybdenum compound, [Mo3(CCH3)2(OAc)6(H2O)3](CF3SO3)2 not only sat on a shelf for 30 years without any sign of degradation but also decomposed in water to generate 2-butyne, which is the coupling adduct of its two ethylidyne ligands. This, according to the researchers, opens another way for aqueous organometallic chemistry.[19]
One method for palladium-catalyzed cross-coupling reactions of aryl halides with fluorinated arenes was reported by Keith Fagnou and co-workers. It is unusual in that it involves C-H functionalisation at an electron deficient arene.[20]

Applications
Many coupling reactions have found their way into pharmaceutical industry [21] and into conjugated organic materials [22]
References
- ^ Organic Synthesis using Transition Metals Rod Bates ISBN 978-1-84127-107-1
- ^ New Trends in Cross-Coupling: Theory and Applications Thomas Colacot (Editor) 2014 ISBN 978-1-84973-896-5
- ^ "The Nobel Prize in Chemistry 2010 - Richard F. Heck, Ei-ichi Negishi, Akira Suzuki". NobelPrize.org. 2010-10-06. Retrieved 2010-10-06.
- ^ Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize Dr. Carin C. C. Johansson Seechurn, Dr. Matthew O. Kitching, Dr. Thomas J. Colacot, Prof. Victor Snieckus Angew. Chem. Int. Ed. 2012, 51, 5062-5085. doi:10.1002/anie.201107017
- ^ Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 1-891389-53-X
- ^ V. P. Ananikov, D. G. Musaev, K. Morokuma, “Theoretical Insight into the C-C Coupling Reactions of the Vinyl, Phenyl, Ethynyl, and Methyl Complexes of Palladium and Platinum” Organometallics 2005, 24, 715. doi:10.1021/om0490841
- ^ a b Benny Bogoslavsky, Ophir Levy, Anna Kotlyar, Miri Salem, Faina Gelman and Avi Bino (2012). "Do Carbyne Radicals Really Exist in Aqueous Solution?". Angewandte Chemie International Edition. 51 (1): 90–94. doi:10.1002/anie.201103652. PMID 22031005.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thayer, Ann (2005-09-05). "Removing Impurities". Chemical & Engineering News. American Chemical Society. Retrieved 2015-12-11.
- ^ Cobalt-Catalyzed Cross-Coupling Reactions Grard Cahiez and Alban Moyeux Chem. Rev., 2010, 110 (3), pp 1435–1462 Publication Date (Web): February 11, 2010 (Review) doi:10.1021/cr9000786
- ^ Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts Lunxiang Yin and Jürgen Liebscher Chem. Rev., 2007, 107 (1), pp 133–173 Publication Date (Web): December 21, 2006 (Article) doi:10.1021/cr0505674
- ^ Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners Ranjan Jana, Tejas P. Pathak, and Matthew S. Sigman Chem. Rev., 2011, 111 (3), pp 1417–1492 doi:10.1021/cr100327p
- ^ Efficient, Selective, and Recyclable Palladium Catalysts in Carbon−Carbon Coupling Reactions rpd Molnr Chem. Rev., 2011, 111 (3), pp 2251–2320 doi:10.1021/cr100355b
- ^ Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds Norio. Miyaura, Akira. Suzuki Chem. Rev., 1995, 95 (7), pp 2457–2483 doi:10.1021/cr00039a007
- ^ Diazonium Salts as Substrates in Palladium-Catalyzed Cross-Coupling Reactions Anna Roglans, Anna Pla-Quintana, and Marcial Moreno-Mañas Chem. Rev., 2006, 106 (11), pp 4622–4643 doi:10.1021/cr0509861
- ^ Nickel-Catalyzed Cross-Couplings Involving Carbon−Oxygen Bonds Brad M. Rosen, Kyle W. Quasdorf, Daniella A. Wilson, Na Zhang, Ana-Maria Resmerita, Neil K. Garg, and Virgil Percec Chem. Rev., 2011, 111 (3), pp 1346–1416 doi:10.1021/cr100259t
- ^ Selected Patented Cross-Coupling Reaction Technologies Jean-Pierre Corbet and Gérard Mignani Chem. Rev., 2006, 106 (7), pp 2651–2710 2006 (Article) doi:10.1021/cr0505268
- ^ Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis Gwilherm Evano, Nicolas Blanchard and Mathieu Toumi Chem. Rev., 2008, 108 (8), pp 3054–3131 doi:10.1021/cr8002505
- ^ Nielsen, Daniel K.; Huang, Chung-Yang (Dennis); Doyle, Abigail G. (2013-08-20). "Directed Nickel-Catalyzed Negishi Cross Coupling of Alkyl Aziridines". Journal of the American Chemical Society. 135 (36): 13605–13609. doi:10.1021/ja4076716. ISSN 0002-7863. Retrieved 2015-12-11.
- ^ A. Bino, M. Ardon and E. Shirman (2005). "Formation of a Carbon-Carbon Triple Bond by Coupling Reactions In Aqueous Solution". Science. 308 (5719): 234–235. Bibcode:2005Sci...308..234B. doi:10.1126/science.1109965. PMID 15821086.
- ^ M. Lafrance, C. N. Rowley, T. K. Woo and K. Fagnou (2006). "Catalytic Intermolecular Direct Arylation of Perfluorobenzenes". J. Am. Chem. Soc. 128 (27): 8754–8756. doi:10.1021/ja062509l. PMID 16819868.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ R.H. Crabtree, The Organometallic Chemistry of the Transition Metals 4th Ed.
- ^ Organotransition Metal Chemistry: From Bonding to Catalysis John Hartwig
