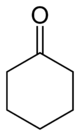
Cyclohexanone
| |||

| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.302 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H10O | |||
| Molar mass | 98.15 g/mol | ||
| Appearance | Colorless, liquid | ||
| Density | 0.9478 g/mL, liquid | ||
| Melting point | −16.4 °C | ||
| Boiling point | 155.65 °C | ||
| Slightly soluble | |||
| Solubility in ethanol | Miscible | ||
Refractive index (nD)
|
1.447 | ||
| Viscosity | 2.02 cP at 25 °C[3] | ||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
+229.03 J.K−1.mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−270.7 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−3519.3 kJmol−1 | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 44 °C | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation. Cyclohexanone is slightly soluble in water (5-10 g/100 mL), but miscible with common organic solvents. Billions of kilograms are produced annually, mainly as a precursor to nylon.[4]
Production
Cyclohexanone is produced by the oxidation of cyclohexane in air, typically using cobalt catalysts:[4]
- C6H12 + O2 → (CH2)5CO + H2O
This process co-forms cyclohexanol, and this mixture, called "KA oil" for ketone-alcohol oil, is the main feedstock for the production of adipic acid. The oxidation involves radicals and the intermediacy of the hydroperoxide C6H11O2H. In some cases, purified cyclohexanol, obtained by hydration of cyclohexene, is the precursor. Alternatively, cyclohexanone can be produced by the partial hydrogenation of phenol:
- C6H5OH + 2 H2 → (CH2)5CO
This process can also be adjusted to favor the formation of cyclohexanol.[4]
Laboratory methods
Cyclohexanone can be prepared from cyclohexanol by oxidation with chromic oxide.
Uses
The great majority of cyclohexanone is consumed in the production of precursors to Nylon 6,6 and Nylon 6. About half of the world's supply is converted to adipic acid, one of two precursors for nylon 6,6. For this application, the KA oil (see above) is oxidized with nitric acid. The other half of the cyclohexanone supply is converted to the oxime. In the presence of sulfuric acid catalyst, the oxime rearranges to caprolactam, a precursor to nylon 6:[4]
Safety
Like cyclohexanol, cyclohexanone is not carcinogenic and is only moderately toxic, with a TLV of 25 ppm for the vapor. It is an irritant.[4]
A recent study of plastic tubing used in medical procedures that circulate blood outside the body suggests a link between this compound and decreased heart function, swelling, loss of taste and short term memory loss.[5]
References
- ^ International Chemical Safety Card 0425
- ^ NIOSH Pocket Guide to Chemical Hazards
- ^ Data extract from Landolt-Börnstein IV/25: Viscosity of Pure Organic Liquids and Binary Liquid Mixtures
- ^ a b c d e Michael T. Musser "Cyclohexanol and Cyclohexanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a08_217
- ^ http://www.eurekalert.org/pub_releases/2009-05/jhmi-cfi043009.php