Ethanol (data page)
Appearance
(Redirected from Ethyl alcohol (data page))
This page provides supplementary chemical data on ethanol.
Material Safety Data Sheet
[edit]
Structure and properties
[edit]| Structure and properties | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index of refraction, n25 | 1.361 | ||||||||||||||||||||||||||||||||||||||
| Dielectric constant, εr | 24.3 ε0 at 20 °C | ||||||||||||||||||||||||||||||||||||||
| Bond strength | ? | ||||||||||||||||||||||||||||||||||||||
| Bond length | ? | ||||||||||||||||||||||||||||||||||||||
| Bond angle | ? | ||||||||||||||||||||||||||||||||||||||
| Magnetic susceptibility[1] | 5.8×10−7 (cgs units, volume) | ||||||||||||||||||||||||||||||||||||||
| Surface tension | 22.39 dyn/cm at 25 °C | ||||||||||||||||||||||||||||||||||||||
| Thermal conductivity[2] | 0.1660 W m−1 K−1 (saturated liquid at 300 K) | ||||||||||||||||||||||||||||||||||||||
| Viscosity[3] |
| ||||||||||||||||||||||||||||||||||||||
Thermodynamic properties
[edit]| Phase behavior | |
|---|---|
| Triple point | 150 K (−123 °C), 0.00043 Pa |
| Critical point | 514 K (241 °C), 63 bar |
| Std enthalpy change of fusion, ΔfusH |
+4.9 kJ/mol |
| Std entropy change of fusion, ΔfusS |
+31 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
+42.3±0.4 kJ/mol[4] |
| Std entropy change of vaporization, ΔvapS |
109.67 J/(mol·K) |
| Molal freezing point constant | −1.99 °C kg/mol |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
−277.7 kJ/mol |
| Standard molar entropy, S |
160.7 J/(mol K)[5] |
| Heat capacity, cp | 111.46 J/(mol K)[5] |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−277.38 kJ/mol |
| Standard molar entropy, S |
159.9 J/(mol K) |
| Enthalpy of combustion, ΔcH |
−1370.7 kJ/mol |
| Heat capacity, cp | 112.4 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−235.3 kJ/mol |
| Standard molar entropy, S |
283 J/(mol K) |
| Heat capacity,[6][7] cp | 78.28 J/(mol K) at 90 °C 87.53 J/(mol K) at 110-220 °C |
| Heat capacity ratio,[6][7] γ = cp/cv |
1.13 at 90 °C |
| van der Waals' constants[8] | a = 1217.9 L2 kPa/mol2 b = 0.08407 L/mol |
Spectral data
[edit]| UV-Vis | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax | ? nm | ||||||||||||||||||||||||||||
| Extinction coefficient, ε | ? | ||||||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||||||
| Major absorption bands[9] |
| ||||||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||||||
| Proton NMR | |||||||||||||||||||||||||||||
| Carbon-13 NMR | http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/IMG.cgi?fname=CDS00245&imgdir=cdsW; | ||||||||||||||||||||||||||||
| Other NMR data | |||||||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||||||
| Masses of main fragments |
|||||||||||||||||||||||||||||
Vapor pressure of liquid
[edit]| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1,520 | 3,800 | 7,600 | 15,200 | 30,400 | 45,600 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P in Pa | 133.3 | 1,333 | 5,333 | 13,332 | 53,329 | 101,325 | 202,650 | 506,625 | 1,013,250 | 2,026,500 | 4,053,000 | 6,079,501 |
| T in °C | −31.3 | −2.3 | 19.0 | 34.9 | 63.5 | 78.4 | 97.5 | 126.0 | 151.8 | 183.0 | 218.0 | 242.0 |
| T in K | 241.85 | 270.85 | 292.15 | 308.05 | 336.65 | 351.55 | 370.65 | 399.15 | 424.95 | 456.15 | 491.15 | 515.15 |
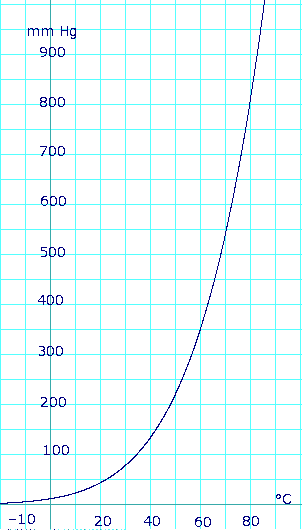 |
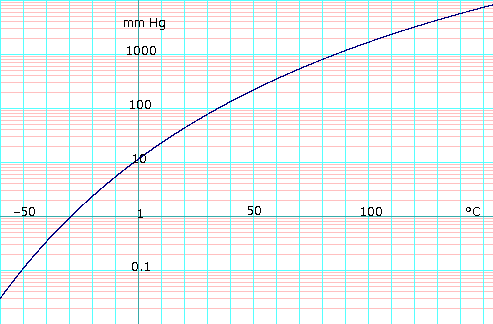 |
Density of ethanol at various temperatures
[edit]Data obtained from Lange 1967
| T °C | ρ, g/cm3 | T °C | ρ, g/cm3 | T °C | ρ, g/cm3 |
|---|---|---|---|---|---|
| 3 | 0.80374 | 16 | 0.79283 | 29 | 0.78182 |
| 4 | 0.80290 | 17 | 0.79198 | 30 | 0.78097 |
| 5 | 0.80207 | 18 | 0.79114 | 31 | 0.78012 |
| 6 | 0.80123 | 19 | 0.79029 | 32 | 0.77927 |
| 7 | 0.80039 | 20 | 0.78945 | 33 | 0.77841 |
| 8 | 0.79956 | 21 | 0.78860 | 34 | 0.77756 |
| 9 | 0.79872 | 22 | 0.78775 | 35 | 0.77671 |
| 10 | 0.79788 | 23 | 0.78691 | 36 | 0.77585 |
| 11 | 0.79704 | 24 | 0.78606 | 37 | 0.77500 |
| 12 | 0.79620 | 25 | 0.78522 | 38 | 0.77414 |
| 39 | 0.77329 | 40 | 0.77244 |
These data correlate as ρ [g/cm3] = −8.461834×10−4 T [°C] + 0.8063372 with an R2 = 0.99999.
Properties of aqueous ethanol solutions
[edit]Data obtained from Lange 1967
| Mass fraction, % |
Volume concentration, % |
Mass concentration, g/(100 ml) at 15.56 °C | Density relative to 4 °C water [citation needed] | Density at 20 °C relative to 20 °C water | Density at 25 °C relative to 25 °C water | Freezing temperature, °C | |||
|---|---|---|---|---|---|---|---|---|---|
| 10 °C | 20 °C | 25 °C | 30 °C | ||||||
| 0.0 | 0.0 | 0.0 | 0.99973 | 0.99823 | 0.99708 | 0.99568 | 1.00000 | 1.00000 | 0 |
| 1.0 | 0.99785 | 0.99636 | 0.99520 | 0.99379 | 0.99813 | 0.99811 | |||
| 2.0 | 0.99602 | 0.99453 | 0.99336 | 0.99194 | 0.99629 | 0.99627 | |||
| 2.5 | 3.13 | 0.99363 | −1 | ||||||
| 3.0 | 0.99426 | 0.99275 | 0.99157 | 0.99014 | 0.99451 | 0.99447 | |||
| 4.0 | 5.00 | 3.97 | 0.99258 | 0.99103 | 0.98984 | 0.98839 | 0.99279 | 0.99274 | |
| 4.8 | 6.00 | 4.76 | 0.98971 | −2 | |||||
| 5.0 | 0.99098 | 0.98938 | 0.98817 | 0.98670 | 0.99113 | 0.99106 | |||
| 5.05 | 6.30 | 5.00 | 0.98930 | ||||||
| 6.0 | 0.98946 | 0.98780 | 0.98656 | 0.98507 | 0.98955 | 0.98945 | |||
| 6.8 | 8.47 | 0.98658 | −3 | ||||||
| 7.0 | 0.98801 | 0.98627 | 0.98500 | 0.98347 | 0.98802 | 0.98788 | |||
| 8.0 | 0.98660 | 0.98478 | 0.98346 | 0.98189 | 0.98653 | 0.98634 | |||
| 9.0 | 0.98524 | 0.98331 | 0.98193 | 0.98031 | 0.98505 | 0.98481 | |||
| 10.0 | 12.40 | 9.84 | 0.98393 | 0.98187 | 0.98043 | 0.97875 | 0.98361 | 0.98330 | |
| 11.0 | 0.98267 | 0.98047 | 0.97897 | 0.97723 | 0.98221 | 0.98184 | |||
| 11.3 | 14.0 | 11.11 | 0.98006 | −5 | |||||
| 12.0 | 15.0 | 0.98145 | 0.97910 | 0.97753 | 0.97573 | 0.98084 | 0.98039 | ||
| 13.0 | 0.98026 | 0.97775 | 0.97611 | 0.97424 | 0.97948 | 0.97897 | |||
| 13.78 | 17.00 | 13.49 | −6.1 | ||||||
| 14.0 | 0.97911 | 0.97643 | 0.97472 | 0.97278 | 0.97816 | 0.97757 | |||
| 15.0 | 0.97800 | 0.97514 | 0.97334 | 0.97133 | 0.97687 | 0.97619 | |||
| 15.02 | 18.50 | 14.68 | 0.97511 | ||||||
| 16.0 | 0.97692 | 0.97387 | 0.97199 | 0.96990 | 0.97560 | 0.97484 | |||
| 16.4 | 20.2 | 0.97336 | −7.5 | ||||||
| 17.0 | 0.97583 | 0.97259 | 0.97062 | 0.96844 | 0.97431 | 0.97346 | |||
| 17.5 | 21.5 | 0.97194 | −8.7 | ||||||
| 18.0 | 22.10 | 17.54 | 0.97473 | 0.97129 | 0.96923 | 0.96697 | 0.97301 | 0.97207 | |
| 18.8 | 23.1 | 0.97024 | −9.4 | ||||||
| 19.0 | 0.97363 | 0.96997 | 0.96782 | 0.96547 | 0.97169 | 0.97065 | |||
| 20.0 | 0.97252 | 0.96864 | 0.96639 | 0.96395 | 0.97036 | 0.96922 | |||
| 20.01 | 24.50 | 19.44 | 0.96863 | ||||||
| 20.3 | 24.8 | 0.96823 | −10.6 | ||||||
| 21.0 | 0.97139 | 0.96729 | 0.96495 | 0.96242 | 0.96901 | 0.96778 | |||
| 22.0 | 0.97024 | 0.96592 | 0.96348 | 0.96087 | 0.96763 | 0.96630 | |||
| 22.11 | 27.00 | 21.43 | 0.96578 | −12.2 | |||||
| 23.0 | 0.96907 | 0.96453 | 0.96199 | 0.95929 | 0.96624 | 0.96481 | |||
| 24.0 | 0.96787 | 0.96312 | 0.96048 | 0.95769 | 0.96483 | 0.96329 | |||
| 24.2 | 29.5 | 0.96283 | −14.0 | ||||||
| 25.0 | 30.40 | 24.12 | 0.96665 | 0.96168 | 0.95895 | 0.95607 | 0.96339 | 0.96176 | |
| 26.0 | 0.96539 | 0.96020 | 0.95738 | 0.95422 | 0.96190 | 0.96018 | |||
| 26.7 | 32.4 | 0.95914 | −16.0 | ||||||
| 27.0 | 0.96406 | 0.95867 | 0.95576 | 0.95272 | 0.96037 | 0.95856 | |||
| 28.0 | 33.90 | 26.90 | 0.96268 | 0.95710 | 0.95410 | 0.95098 | 0.95880 | 0.95689 | |
| 29.0 | 0.96125 | 0.95548 | 0.95241 | 0.94922 | 0.95717 | 0.95520 | |||
| 29.9 | 36.1 | 0.95400 | −18.9 | ||||||
| 30.0 | 36.20 | 28.73 | 0.95977 | 0.95382 | 0.95067 | 0.94741 | 0.95551 | 0.95345 | |
| 31.0 | 0.95823 | 0.95212 | 0.94890 | 0.94557 | 0.95381 | 0.95168 | |||
| 32.0 | 0.95665 | 0.95038 | 0.94709 | 0.94370 | 0.95207 | 0.94986 | |||
| 33.0 | 0.95502 | 0.94860 | 0.94525 | 0.94180 | 0.95028 | 0.94802 | |||
| 33.8 | 40.5 | 0.94715 | −23.6 | ||||||
| 34.0 | 0.95334 | 0.94679 | 0.94337 | 0.93986 | 0.94847 | 0.94613 | |||
| 35.0 | 0.95162 | 0.94494 | 0.94146 | 0.93790 | 0.94662 | 0.94422 | |||
| 35.04 | 41.90 | 33.25 | 0.94486 | ||||||
| 36.0 | 0.94986 | 0.94306 | 0.93952 | 0.93591 | 0.94473 | 0.94227 | |||
| 37.0 | 0.94805 | 0.94114 | 0.93756 | 0.93390 | 0.94281 | 0.94031 | |||
| 38.0 | 0.94620 | 0.93919 | 0.93556 | 0.93186 | 0.94086 | 0.93830 | |||
| 39.0 | 46.3 | 0.94431 | 0.93720 | 0.93353 | 0.92979 | 0.93886 | 0.93626 | −28.7 | |
| 40.0 | 0.94238 | 0.93518 | 0.93148 | 0.92770 | 0.93684 | 0.93421 | |||
| 40.04 | 47.40 | 37.61 | 0.93510 | ||||||
| 41.0 | 0.94042 | 0.93314 | 0.92940 | 0.92558 | 0.93479 | 0.93212 | |||
| 42.0 | 0.93842 | 0.93107 | 0.92729 | 0.92344 | 0.93272 | 0.93001 | |||
| 43.0 | 0.93639 | 0.92897 | 0.92516 | 0.92128 | 0.93062 | 0.92787 | |||
| 44.0 | 0.93433 | 0.92685 | 0.92301 | 0.91910 | 0.92849 | 0.92571 | |||
| 45.0 | 0.93226 | 0.92472 | 0.92085 | 0.91692 | 0.92636 | 0.92355 | |||
| 45.31 | 53.00 | 42.07 | 0.92406 | ||||||
| 46.0 | 0.93017 | 0.92257 | 0.91868 | 0.91472 | 0.92421 | 0.92137 | |||
| 46.3 | 53.8 | 0.92193 | −33.9 | ||||||
| 47.0 | 0.92806 | 0.92041 | 0.91649 | 0.91250 | 0.92204 | 0.91917 | |||
| 48.0 | 0.92593 | 0.91823 | 0.91429 | 0.91028 | 0.91986 | 0.91697 | |||
| 49.0 | 0.92379 | 0.91604 | 0.91208 | 0.90805 | 0.91766 | 0.91475 | |||
| 50.0 | 0.92162 | 0.91384 | 0.90985 | 0.90580 | 0.91546 | 0.91251 | |||
| 50.16 | 58.0 | 46.04 | 0.91349 | ||||||
| 51.0 | 0.91943 | 0.91160 | 0.90760 | 0.90353 | 0.91322 | 0.91026 | |||
| 52.0 | 0.91723 | 0.90936 | 0.90524 | 0.90125 | 0.91097 | 0.90799 | |||
| 53.0 | 0.91502 | 0.90711 | 0.90307 | 0.89896 | 0.90872 | 0.90571 | |||
| 54.0 | 0.91279 | 0.90485 | 0.90079 | 0.89667 | 0.90645 | 0.90343 | |||
| 55.0 | 0.91055 | 0.90258 | 0.89850 | 0.89437 | 0.90418 | 0.90113 | |||
| 55.16 | 63.0 | 50.00 | 0.90220 | ||||||
| 56.0 | 0.90831 | 0.90031 | 0.89621 | 0.89206 | 0.90191 | 0.89833 | |||
| 56.1 | 63.6 | 0.90008 | −41.0 | ||||||
| 57.0 | 0.90607 | 0.89803 | 0.89392 | 0.88975 | 0.89962 | 0.89654 | |||
| 58.0 | 0.90381 | 0.89574 | 0.89162 | 0.88744 | 0.89733 | 0.89423 | |||
| 59.0 | 0.90154 | 0.89344 | 0.88931 | 0.88512 | 0.89502 | 0.89191 | |||
| 60.0 | 0.89927 | 0.89113 | 0.88699 | 0.88278 | 0.89271 | 0.88959 | |||
| 60.33 | 68.0 | 53.98 | 0.89038 | ||||||
| 61.0 | 0.89698 | 0.88882 | 0.88466 | 0.88044 | 0.89040 | 0.88725 | |||
| 62.0 | 0.89468 | 0.88650 | 0.88233 | 0.87809 | 0.88807 | 0.88491 | |||
| 63.0 | 0.89237 | 0.88417 | 0.87998 | 0.87574 | 0.88574 | 0.88256 | |||
| 64.0 | 0.89006 | 0.88183 | 0.87763 | 0.87337 | 0.88339 | 0.88020 | |||
| 65.0 | 0.88774 | 0.87948 | 0.87527 | 0.87100 | 0.88104 | 0.87783 | |||
| 66.0 | 0.88541 | 0.87713 | 0.87291 | 0.86863 | 0.87869 | 0.87547 | |||
| 67.0 | 0.88308 | 0.87477 | 0.87054 | 0.86625 | 0.87632 | 0.87309 | |||
| 68.0 | 0.88071 | 0.87241 | 0.86817 | 0.86387 | 0.87396 | 0.87071 | |||
| 69.0 | 0.87839 | 0.87004 | 0.86579 | 0.86148 | 0.87158 | 0.86833 | |||
| 70.0 | 0.87602 | 0.86766 | 0.86340 | 0.85908 | 0.86920 | 0.86593 | |||
| 71.0 | 0.87365 | 0.86527 | 0.86100 | 0.85667 | 0.86680 | 0.86352 | |||
| 71.9 | 78.3 | 0.86311 | −51.3 | ||||||
| 72.0 | 0.87127 | 0.86287 | 0.85859 | 0.85426 | 0.86440 | 0.86110 | |||
| 73.0 | 0.86888 | 0.86047 | 0.85618 | 0.85184 | 0.86200 | 0.85869 | |||
| 74.0 | 0.86648 | 0.85806 | 0.85376 | 0.84941 | 0.85958 | 0.85626 | |||
| 75.0 | 0.86408 | 0.85564 | 0.85135 | 0.84698 | 0.85716 | 0.85383 | |||
| 76.0 | 0.86168 | 0.85322 | 0.84891 | 0.84455 | 0.85473 | 0.85140 | |||
| 77.0 | 0.85927 | 0.85079 | 0.84647 | 0.84211 | 0.85230 | 0.84895 | |||
| 78.0 | 0.85685 | 0.84835 | 0.84403 | 0.83966 | 0.84985 | 0.84650 | |||
| 79.0 | 0.85422 | 0.84590 | 0.84158 | 0.83720 | 0.84740 | 0.84404 | |||
| 80.0 | 0.85197 | 0.84344 | 0.83911 | 0.83473 | 0.84494 | 0.84157 | |||
| 81.0 | 0.84950 | 0.84096 | 0.83664 | 0.83224 | 0.84245 | 0.83909 | |||
| 82.0 | 0.84702 | 0.83848 | 0.83415 | 0.82974 | 0.83997 | 0.83659 | |||
| 83.0 | 0.84453 | 0.83599 | 0.83164 | 0.82724 | 0.83747 | 0.83408 | |||
| 84.0 | 0.84203 | 0.83348 | 0.82913 | 0.82473 | 0.83496 | 0.83156 | |||
| 85.0 | 0.83951 | 0.83095 | 0.82660 | 0.82220 | 0.83242 | 0.82902 | |||
| 86.0 | 0.83697 | 0.82840 | 0.82405 | 0.81965 | 0.82987 | 0.82646 | |||
| 87.0 | 0.83441 | 0.82583 | 0.82148 | 0.81708 | 0.82729 | 0.82389 | |||
| 88.0 | 0.83181 | 0.82323 | 0.81888 | 0.81448 | 0.82469 | 0.82128 | |||
| 89.0 | 0.82919 | 0.82062 | 0.81626 | 0.81186 | 0.82207 | 0.81865 | |||
| 90.0 | 0.82654 | 0.81797 | 0.81362 | 0.80922 | 0.81942 | 0.81600 | |||
| 91.00 | 94.00 | 74.62 | 0.82386 | 0.81529 | 0.81094 | 0.80655 | 0.81674 | 0.81331 | |
| 92.0 | 0.82114 | 0.81257 | 0.80823 | 0.80384 | 0.81401 | 0.81060 | |||
| 93.0 | 0.81839 | 0.80983 | 0.80549 | 0.80111 | 0.81127 | 0.80785 | |||
| 94.0 | 0.81561 | 0.80705 | 0.80272 | 0.79835 | 0.80848 | 0.80507 | |||
| 95.0 | 0.81278 | 0.80424 | 0.79991 | 0.79555 | 0.80567 | 0.80225 | |||
| 96.0 | 0.80991 | 0.80138 | 0.79706 | 0.79271 | 0.80280 | 0.79939 | |||
| 97.0 | 0.80698 | 0.79846 | 0.79415 | 0.78981 | 0.79988 | 0.79648 | |||
| 98.0 | 0.80399 | 0.79547 | 0.79117 | 0.78684 | 0.79688 | 0.79349 | |||
| 99.0 | 0.80094 | 0.79243 | 0.78814 | 0.78382 | 0.79383 | 0.79045 | |||
| 100.0 | 100.0 | 79.39 | 0.79784 | 0.78934 | 0.78506 | 0.78075 | 0.79074 | 0.78736 | −114.3 |
| Mass fraction, % |
Volume concentration, % |
Mass concentration, g/(100 ml) at 15.56 °C | Density relative to 4 °C water | Density at 20 °C relative to 20 °C water | Density at 25 °C relative to 25 °C water | Freezing temperature, °C | |||
| 10 °C | 20 °C | 25 °C | 30 °C | ||||||
Boiling points of aqueous solutions
[edit]Data obtained from CRC Handbook of Chemistry (Page 2117)[7]: 2391
| BP °C | Weight % ethanol | BP °C | Weight % ethanol | |||
| liquid | vapor | liquid | vapor | |||
| 78.1 | 95.5‡ | 95.5‡ | ||||
| 78.2 | 91 | 92 | 86.5 | 18 | 71 | |
| 78.4 | 85 | 89 | 87.0 | 17 | 70 | |
| 78.6 | 82 | 88 | 87.5 | 16 | 69 | |
| 78.8 | 80 | 87 | 88.0 | 15 | 68 | |
| 79.0 | 78 | 86 | 88.5 | 13 | 67 | |
| 79.2 | 76 | 85 | 89.0 | 12 | 65 | |
| 79.4 | 74 | 85 | 89.5 | 11 | 63 | |
| 79.6 | 72 | 84 | 90.0 | 10 | 61 | |
| 79.8 | 69 | 84 | 90.5 | 10 | 59 | |
| 80.0 | 67 | 83 | 91.0 | 9 | 57 | |
| 80.2 | 64 | 83 | 91.5 | 8 | 55 | |
| 80.4 | 62 | 82 | 92.0 | 8 | 53 | |
| 80.6 | 59 | 82 | 92.5 | 7 | 51 | |
| 80.8 | 56 | 81 | 93.0 | 6 | 49 | |
| 81.0 | 53 | 81 | 93.5 | 6 | 46 | |
| 81.2 | 50 | 80 | 94.0 | 5 | 44 | |
| 81.4 | 47 | 80 | 94.5 | 5 | 42 | |
| 81.6 | 45 | 80 | 95.0 | 4 | 39 | |
| 81.8 | 43 | 79 | 95.5 | 4 | 36 | |
| 82.0 | 41 | 79 | 96.0 | 3 | 33 | |
| 82.5 | 36 | 78 | 96.5 | 3 | 30 | |
| 83.0 | 33 | 78 | 97.0 | 2 | 27 | |
| 83.5 | 30 | 77 | 97.5 | 2 | 23 | |
| 84.0 | 27 | 77 | 98.0 | 1 | 19 | |
| 84.5 | 25 | 75 | 98.5 | 1 | 15 | |
| 85.0 | 23 | 74 | 99.0 | < 1 | 10 | |
| 85.5 | 21 | 73 | 99.5 | < 1 | 5 | |
| 86.0 | 20 | 72 | 100.0 | 0 | 0 | |
Charts
[edit]
|

|
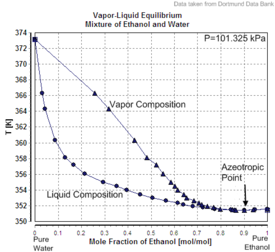
|
| Excess volume of the mixture of ethanol and water (volume contraction) | Heat of mixing of the mixture of ethanol and water | Vapor–liquid equilibrium of the mixture of ethanol and water (including azeotrope) |

|

|
|
| Solid–liquid equilibrium of the mixture of ethanol and water (including eutecticum) | Miscibility gap in the mixture of dodecane and ethanol |
This box:
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
References
[edit]- ^ NMR-002: Sample Devices and Magnetic Susceptibility
- ^ Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter – the TPRC data series. Volume 3. Thermal conductivity – nonmetallic liquids and gases. Data book. 1970.
- ^ "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 12 May 2007.
- ^ "Ethanol". webbook.nist.gov. Retrieved 7 December 2021.
- ^ a b Atkins, Peter; de Paula, Julio (2010). Atkins' Physical Chemistry (9th ed.). Oxford: OUP. pp. 913–947. ISBN 978-0-19-954337-3.
- ^ a b Lange 1967, pp. 1525–1528.
- ^ a b c Hodgman, Charles D; Weast, Robert C; Shankland, Robert S; Selby, Samuel M (1963). CRC Handbook of chemistry and physics : a ready-reference book of chemical and physical data (44th ed.). Cleveland Ohio: The Chemical Rubber Publishing. pp. 2582–2584.
- ^ Lange 1967, pp. 1522–1524.
- ^ "Spectral Database for Organic Compounds" (Queriable database). Advanced Industrial Science and Technology. Retrieved 9 June 2007.
- ^ Lange 1967.
- Lange, Norbert Adolph (1967). John Aurie Dean (ed.). Lange's Handbook of Chemistry (10th ed.). McGraw-Hill.
- Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD)


