Helicene

In organic chemistry, helicenes are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped molecules. The chemistry of helicenes has attracted continuing attention because of their unique structural, spectral, and optical features.[1] [2] [3] [4] [5] [6] [7]
Structure
For helicenes with 6 benzene units a 360° turn is completed. In the helicene series the dihedral angles between the extremities increases going from [4]helicene (26°) to [6]helicene (58°) and then decreases again for example in [7]helicene (30°).
Properties
Helicenes are notable for having chirality despite lacking both asymmetric carbons and chiral centers. The chirality results from the handedness of the helicity itself. The clockwise and counterclockwise helices are non-superposable as a result of their axial chirality. By convention a left-handed helix is minus and labeled M, a right-handed helix is plus and labeled P. Evidence from CD spectroscopy suggests left-handed helices are levorotatory and right-handed helices are dextrorotatory.
Synthesis
The first helicene structure was reported by Jakob Meisenheimer in 1903 as the reduction product of 2-nitronaphthalene.[8] [5]helicene was synthesised in 1918 by Weitzenböck & Klingler.[9] The first [6]helicene (also called hexahelicene) was synthesized by M. S. Newman and D. Lednicer in 1955 via a scheme that closed the two central rings by Friedel–Crafts cyclization of carboxylic acid compounds.[10] [11] Since then, several methods for synthesizing helicenes with different lengths and substituents are used. The oxidative photocyclization of a stilbene-type precursor is used most often as the key step. The longest helicene, [14]helicene, was prepared in 1975 by this method.
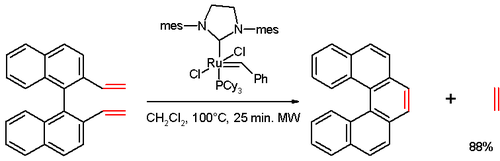
In one study,[12] [5]helicene was synthesized in an olefin metathesis reaction of a divinyl compound (prepared from 1,1'-bi-2-naphthol (BINOL) in several steps), with Grubbs' second generation catalyst:
Other approach is also non-photochemical and is based on assembly of biphenylyl-naphthalenes and their platinum-catalyzed double cycloisomerization leading to various [6]helicenes: [13]
Polyacenes are the linear 1,3-fused or meta-analogs of helicenes. Conceptually related compounds are the circulenes.
-
[4]Helicene
-
[5]Helicene
-
[6]Helicene
-
[6]Helicene, other chirality
-
[7]Helicene
-
[7]Helicene, other chirality
-
[8]Helicene
-
[9]Helicene
-
[10]Helicene
-
[11]Helicene
-
[12]Helicene
-
[13]Helicene
-
[14]Helicene
-
[15]Helicene
-
[16]Helicene
-
[18]Helicene
See also
References
- ^ Martin, R. H. (1974), The Helicenes. Angew. Chem. Int. Ed. Engl., 13: 649–660. doi:10.1002/anie.197406491
- ^ Helicenes: Synthesis and Applications Yun Shen and Chuan-Feng Chen Chemical Reviews Article ASAP doi:10.1021/cr200087r
- ^ Diels–Alder Additions of Benzynes within Helicene Skeletons David Zhigang Wang, Thomas J. Katz, James Golen, and Arnold L. Rheingold J. Org. Chem.; 2004; 69(22) pp 7769–7771 doi:10.1021/jo048707h
- ^ One hundred years of helicene chemistry. Part 1: non-stereoselective syntheses of carbohelicenes Marc Gingras Chem. Soc. Rev., 2013,42, 968-1006 doi:10.1039/C2CS35154D
- ^ One hundred years of helicene chemistry. Part 2: stereoselective syntheses and chiral separations of carbohelicenes Marc Gingras, Guy Félix and Romain Peresutti Chem. Soc. Rev., 2013,42, 1007-1050 doi:10.1039/C2CS35111K
- ^ One hundred years of helicene chemistry. Part 3: applications and properties of carbohelicenes Marc Gingras Chem. Soc. Rev., 2013,42, 1051-1095 doi:10.1039/C2CS35134J
- ^ Recent Development of Helicene Synthesis Ken Kamikawa Journal of Synthetic Organic Chemistry, Japan Vol. 72 (2014) No. 1 p. 58-67 doi:10.5059/yukigoseikyokaishi.72.58
- ^ Meisenheimer, J. and Witte, K. (1903), Reduction von 2-Nitronaphtalin. Berichte der deutschen chemischen Gesellschaft, 36: 4153–4164. doi:10.1002/cber.19030360481
- ^ Synthese der isomeren Kohlenwasserstoffe 1, 2–5, 6-Dibenzanthracen und 3, 4–5, 6-Dibenzphenanthren Richard Weitzenböck and Albert Klingler Monatshefte für Chemie / Chemical Monthly Volume 39, Number 5, 315–323, doi:10.1007/BF01524529
- ^ A NEW REAGENT FOR RESOLUTION BY COMPLEX FORMATION; THE RESOLUTION OF PHENANTHRO-[3,4-c]PHENANTHRENE Melvin S. Newman, Wilson B. Lutz, and Daniel Lednicer Journal of the American Chemical Society 1955 77 (12), 3420–3421 doi:10.1021/ja01617a097
- ^ The Synthesis and Resolution of Hexahelicene Melvin S. Newman and Daniel Lednicer Journal of the American Chemical Society 1956 78 (18), 4765–4770 doi:10.1021/ja01599a060
- ^ Preparation of Helicenes through Olefin Metathesis Shawn K. Collins, Alain Grandbois, Martin P. Vachon, Julie Côté Angewandte Chemie International Edition Volume 45, Issue 18 , Pages 2923–2926 2006 doi:10.1002/anie.200504150
- ^ Synthesis of Hexahelicene and 1-Methoxyhexahelicene via Cycloisomerization of Biphenylyl-Naphthalene Derivatives. Storch J., Sýkora J., Čermák J., Karban J., Císařová I., Růžička A. J. Org. Chem. 2009, 74, 3090. doi: 10.1021/jo900077j


![[4]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/e/ee/Tetrahelicene.jpg/120px-Tetrahelicene.jpg)
![[5]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/7/7c/Pentahelicene.jpg/120px-Pentahelicene.jpg)
![[6]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/9/95/Hexahelicene.jpg/120px-Hexahelicene.jpg)
![[6]Helicene, other chirality](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e2/Hexahelicene2.jpg/119px-Hexahelicene2.jpg)
![[7]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/2/29/Heptahelicene.jpg/120px-Heptahelicene.jpg)
![[7]Helicene, other chirality](http://upload.wikimedia.org/wikipedia/commons/thumb/9/9f/Heptahelicene2.jpg/120px-Heptahelicene2.jpg)
![[8]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/6/64/Octahelicene.jpg/120px-Octahelicene.jpg)
![[9]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/5/57/Nonahelicene.jpg/120px-Nonahelicene.jpg)
![[10]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Decahelicene.jpg/90px-Decahelicene.jpg)
![[11]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/2/2e/Undecahelicene.jpg/120px-Undecahelicene.jpg)
![[12]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e5/Dodecahelicene.jpg/111px-Dodecahelicene.jpg)
![[13]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/0/08/Tridecahelicene.jpg/99px-Tridecahelicene.jpg)
![[14]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/8/86/Tetradecahelicene.jpg/120px-Tetradecahelicene.jpg)
![[15]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/c/cc/Pentadecahelicene.jpg/120px-Pentadecahelicene.jpg)
![[16]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e6/Hexadecahelicene.jpg/112px-Hexadecahelicene.jpg)
![[18]Helicene](http://upload.wikimedia.org/wikipedia/commons/thumb/0/04/Octadecahelicene.jpg/97px-Octadecahelicene.jpg)