Hydroxymethylpentylcyclohexenecarboxaldehyde
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|

| |
| Names | |
|---|---|
| IUPAC name
4-(4-Hydroxy-4-methylpentyl)-1-cyclohex-3-enecarboxaldehyde
| |
| Other names
Lyral, Kovanol, Mugonal, Landolal
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.046.225 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H22O2 | |
| Molar mass | 210.317 g·mol−1 |
| Density | 0.995 g/mL at 20 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydroxymethylpentylcyclohexenecarboxaldehyde is a synthetic fragrance known by the trade names Lyral, Kovanol, Mugonal, Landolal. It is found in some soaps, eau de toilettes, aftershaves and deodorants.
It is listed as an allergen by EU Directive 76/768/EEC.[2]
It is the cause of contact allergic reactions in 2-3% of eczema patients undergoing patch testing.
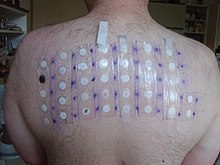
Lyral has been included in the standard patch test series in many clinics due to its importance as an allergen. It has been used without restrictions in cosmetic products, until now. In the present study, the dose-response relationship of Lyral contact allergy was studied with doses relevant for normal exposure in cosmetic products. 18 eczema patients, who previously had given a positive patch test to Lyral 5% petrolatum, were included along with 7 control subjects. All cases were tested with a serial dilution of Lyral in ethanol 6% to 6 p.p.m and subjected to a 2-week, repeated open application test with a low dose of Lyral in ethanol.
In the case of no reaction, this was followed by another 2 weeks of testing with a higher dose. The test was performed at the volar aspect of the forearm. In 16 of 18 cases (89%), a positive use test developed, 11 reacting to the low and 5 to the high concentration. None reacted to the vehicle control of ethanol applied to the contralateral arm. All controls were negative to both the test solutions of Lyral and the ethanol control. The difference between the test and the control group was statistically significant (Fisher's test, P < 0.001). It is concluded that Lyral at the current usage levels is inducing sensitization in the community. The same levels were shown to elicit allergic contact dermatitis in almost all sensitized individuals. A significant reduction in usage concentrations is recommended to prevent contact allergic reactions.
