Hypobromous acid
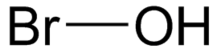
| |
| |
| Names | |
|---|---|
| IUPAC name
hypobromous acid, bromic(I) acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.119.006 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HBrO | |
| Molar mass | 96.911 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hypobromous acid is a weak, unstable acid with chemical formula HBrO. It is also called bromic(I) acid, bromanol or hydroxidobromine. It occurs only in solution and has chemical and physical properties that are very similar to those of hypochlorous acid.
In aqueous solution, hypobromous acid partially dissociates into the hypobromite anion OBr− and the cation H+. Like the acid, hypobromite salts are unstable and when evaporated or boiled to dryness, they undergo a disproportionation reaction, yielding the respective bromate and bromide salts.
When pure bromine is added to water, it forms hypobromous acid and hydrobromic acid (HBr):
- Br2(l) + H2O(l) ↔ HOBr(aq) + HBr (aq)
HOBr is used as a bleach, an oxidizer, a deodorant, and a disinfectant, due to its ability to kill the cells of many pathogens. The compound is generated in warm-blooded vertebrate organisms especially by eosinophils, which produce it by the action of eosinophil peroxidase, an enzyme which preferentially uses bromide.[1] Bromide is also used in hot tubs and spas as a germicidal agent, using the action of an oxidizing agent to generate hypobromite in a similar fashion to the peroxidase in eosinophils. It is especially effective when used in combination with its congener, hypochlorous acid.
References
- ^ Mayeno, AN; Curran, AJ; Roberts, RL; Foote, CS (1989). "Eosinophils preferentially use bromide to generate halogenating agents -- Mayeno et al. 264 (10): 5660 -- Journal of Biological Chemistry". The Journal of Biological Chemistry. 264 (10): 5660–8. PMID 2538427. Retrieved 2008-01-12.
