Lanthanum carbide
| |
| Names | |
|---|---|
| Other names
Dicarbidolanthanum(II)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.923 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| LaC2 | |
| Molar mass | 162.927 g/mol |
| Appearance | tetrahedral crystals |
| Density | 5.29 g/cm3, solid |
| Melting point | 2,360 °C (4,280 °F; 2,630 K) |
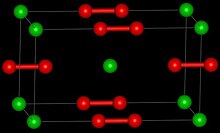
| Structure | |
| Tetragonal | |
| D174h, I4/mmm, tI6 | |
| 6 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lanthanum carbide (LaC2) is a chemical compound. It is being studied in relation to the manufacture of certain types of superconductors and nanotubes.[2]
Preparation
[edit]LaC2 can be prepared by reacting lanthanum oxide, La2O3, with carbon in an electric furnace, or by melting pellets of the elements in an arc furnace.[3]
Properties
[edit]LaC2 reacts with water to form acetylene, C2H2 and a mixture of complex hydrocarbons.[3] LaC2 is a metallic conductor, in contrast to CaC2 which is an insulator.[3] The crystal structure of LaC2 shows that it contains C2 units with a C-C bond length of 130.3 pm, which is longer than the C-C bond length in calcium carbide, 119.2 pm, which is close to that of ethyne.[3] The structure of LaC2 can be described as La3+C22−(e-) where the electron enters the conduction band and antibonding orbitals on the C2 anion, increasing the bond length. This is analogous to the bonding present in the nitridoborate, CaNiBN.[4]
Lanthanum carbide in carbon nano structures
[edit]A method for making macroscopic quantities of C60 and the confirmation of the hollow, cagelike structures was published in 1990 by Kratschmer and co-workers.[5] This was followed by the publication of methods for higher fullerenes (C70 and higher). In 1993, scientists discovered how to make a compound which is not as susceptible to moisture and air. They made containers to hold buckminsterfullerenes, or buckyballs; therefore they nicknamed the containers ‘buckyjars’.[6] A few US patents were issued to universities in the mid-1990s; experiments with manufacturing techniques have continued at universities around the globe, including India, Japan, and Sweden.[7][8]
Lanthanum atoms caged in fullerenes
[edit]In La@C72, the lanthanum appears to stabilize the C72 carbon cage. A 1998 study by Stevenson et al. verified the presence of La@C72 as well as La2@C72, but empty-cage C72 was absent, based on laser desorption mass spectrometry and UV−vis spectroscopy.[9] A 2008 study by Lu et al. showed that La2C72 do not adhere to the isolated pentagon rule (IPR), but has two pairs of fused pentagons at each pole of the cage and that the two La atoms reside close to the two fused-pentagon pairs. This result lends additional support to the idea that the carbon cage is stabilized by the La atoms.[10]
In addition to the properties included in the table at right, the magnetic properties of bulk amounts of La@C82 (isolated from various hollow fullerenes) have been tested. Magnetization data for an isolated La@C82 isomer were obtained using a SQUID magnetometer at temperatures ranging from 3 to 300 K. For La@C82 the inverse susceptibility as a function of temperature was observed to follow a Curie-Weiss law. The effective magnetic moment per La@C82 was found to be 0.38μB.[11]
Lanthanum carbide has also shown superconductive properties when converted into a layered lanthanum carbide halide La2C2X2 (X=Br,I). Investigations using high-resolution neutron powder diffraction measurements from room temperature to 1.5 Kelvin showed that it has superconductive properties at about 7.03 Kelvin for X=Br and at about 1.7 Kelvin for X=I, respectively.[12]
References
[edit]- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 4–64. ISBN 0-8493-0594-2.
- ^ Awasthi, Kalpana; Singh, A. K.; Srivastava, O. N. (2002). "Synthesis and Characterization of Lanthanum Carbide Nanotubes". Journal of Nanoscience and Nanotechnology. 2 (1): 67–71. doi:10.1166/jnn.2002.078. ISSN 1533-4880. PMID 12908323.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 299. ISBN 978-0-08-037941-8.
- ^ Blaschkowski, Björn; Meyer, H.-Jürgen (2002). "Electronic Conditions of Diatomic (BN) Anions in the Structure of CaNiBN". Zeitschrift für anorganische und allgemeine Chemie. 628 (6): 1249. doi:10.1002/1521-3749(200206)628:6<1249::AID-ZAAC1249>3.0.CO;2-S. ISSN 0044-2313.
- ^ Kratschmer W, Lamb, LD, Fostiropoulos K, Huffman, DR, Nature 1990, 347:354; Kratschmer W, Fostiropoulos K, Huffman DR, Chem Phys Lett 1990, 170:167; see also Liu M and Cowley JM, Encapsulation of lanthanum carbide in carbon nanotubules and carbon nanoparticles, Carbon 33(2):225-232, 1995.
- ^ Wellesley Web Page Archived 2006-09-03 at the Wayback Machine
- ^ Awasthu, K; Singh, AK; Srivastava, ON (2002). "Synthesis and characterization of lanthanum carbide nanotubes". Journal of Nanoscience and Nanotechnology. 2 (1): 67–71. doi:10.1166/jnn.2002.078. PMID 12908323.
- ^ Lassesson, A; Gromov, A; Lehlig, K; Taninaka, A; Shinohara, H; Campbell, EEB (2003). "Formation of small lanthanum carbide ions from laser-induced fragmentation of LaatC82". Journal of Chemical Physics. 119 (11): 5591–5600. Bibcode:2003JChPh.119.5591L. doi:10.1063/1.1599833.
- ^ Stevenson, S; Burbank, P; Harich, K; Sun, Z; Dorn, HC (1998). "Metal-mediated stabilization of a carbon cage" (PDF). Journal of Physical Chemistry A. 102 (17): 2833–2837. doi:10.1021/jp980452m. Archived from the original (PDF) on 2019-02-17. Retrieved 2019-07-10.
- ^ Lu, Xing; Nikawa, Hidefumi; Nakahodo, Tsukasa; Tsuchiya, Takahiro; Ishitsuka, Midori O.; Maeda, Yutaka; Akasaka, Takeshi; Toki, Makoto; Sawa, Hiroshi; Slanina, Zdenek; Mizorogi, Naomi; Nagase, Shigeru (2008). "Chemical Understanding of a Non-IPR Metallofullerene: Stabilization of Encaged Metals on Fused-Pentagon Bonds in La2@C72". Journal of the American Chemical Society. 130 (28): 9129–9136. doi:10.1021/ja8019577. ISSN 0002-7863. PMID 18570421.
- ^ Funasaka, H; Sugiyama, K; Yamamoto, K; Takahashi, T (1995). "Magnetic properties of rare-earth metallofullerenes". Journal of Physical Chemistry. 99 (7): 1826–1830. doi:10.1021/j100007a005.
- ^ Ahn, K; Kremer, RK; Mattausch, H; Simon, A (2000). "Superconductivity in layered lanthanum carbide halides". Journal of Alloys and Compounds. 303–304: 257–261. doi:10.1016/s0925-8388(00)00669-1.
External links
[edit]- MIT Open Courseware 3.091 – Introduction to Solid State Chemistry
- 2001 US Patent – Carbide nanomaterials.
- 1997 US Patent – Storage of hydrogen in layered nanostructures.
- 1996 US Patent – Metal, alloy, or metal carbide nanoparticles and a process for forming same.
- 1995 US Patent – Magnetic metal or metal carbide nanoparticles and a process for forming same.
