Litmus


Litmus is a water soluble mixture of different dyes extracted from lichens. It is often absorbed onto filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity.
History
Litmus was used for the first time about 1300 AD by Spanish alchemist Arnaldus de Villa Nova.[1] From the 16th century on, the blue dye was extracted from some lichens, especially in the Netherlands.
Natural sources

Litmus can be found in different species of lichens. The dyes are extracted from such species as Roccella tinctoria (South America), Roccella fuciformis (Angola and Madagascar), Roccella pygmaea (Algeria), Roccella phycopsis, Lecanora tartarea (Norway, Sweden), Variolaria dealbata, Ochrolechia parella, Parmotrema tinctorum, and Parmelia. Currently, the main sources are Roccella montagnei (Mozambique) and Dendrographa leucophoea (California).[1]
Uses
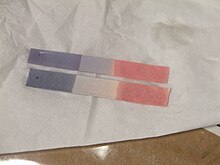
The main use of litmus is to test whether a solution is acidic or basic. Wet litmus paper can also be used to test for water-soluble gases that affect acidity or alkalinity; the gas dissolves in the water and the resulting solution colors the litmus paper. For instance, ammonia gas, which is alkaline, colors the red litmus paper blue.
Blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic or alkaline conditions, with the color change occurring over the pH range 4.5-8.3 at 25 °C (77 °F). Neutral litmus paper is purple.[1] Litmus can also be prepared as an aqueous solution that functions similarly. Under acidic conditions, the solution is red, and under basic conditions, the solution is blue.
| Litmus (pH indicator) | ||
| below pH 4.5 | above pH 8.3 | |
| 4.5 | ⇌ | 8.3 |
Chemical reactions other than acid-base can also cause a color change to litmus paper. For instance, chlorine gas turns blue litmus paper white – the litmus dye is bleached,[2] because of presence of hypochlorite ions. This reaction is irreversible, so the litmus is not acting as an indicator in this situation.
Chemistry
The litmus mixture has the CAS number 1393-92-6 and contains 10 to 15 different dyes. Most of the chemical components of litmus are likely to be the same as those of the related mixture known as orcein, but in different proportions. In contrast with orcein, the principal constituent of litmus has an average molecular weight of 3300.[3] Acid-base indicators on litmus owe their properties to a 7-hydroxyphenoxazone chromophore.[4] Some fractions of litmus were given specific names including erythrolitmin (or erythrolein), azolitmin, spaniolitmin, leucoorcein, and leucazolitmin. Azolitmin shows nearly the same effect as litmus.[5]
Mechanism
Red litmus contains a weak diprotic acid. When it is exposed to a basic compound, the hydrogen ions react with the added base. The conjugated base, formed from the litmus acid, has a blue color, so the wet red litmus paper turns blue in alkaline solution.
See also
- Nitrazine strips - measures pH about 4.5 to 7.5 with more precision
- Phenolphthalein
- Bromothymol blue
- Methyl orange
- Universal indicator - modern paper measures pH 1 to 14 with distinct colors for each. The color chart is usually included with the strips.
References
- ^ a b c Manfred Neupert: Lackmus in Römpp Lexikon Chemie (German), January 31, 2013.
- ^ UCC - Chlorine
- ^ Beecken, H.; E-M. Gottschalk; U. v Gizycki; H. Krämer; D. Maassen; H-G. Matthies; H. Musso; C. Rathjen; Ul. Zdhorszky (2003). "Orcein and Litmus". Biotechnic & Histochemistry. 78 (6): 289–302. doi:10.1080/10520290410001671362.
- ^ H. Musso, C. Rathjen. "Orcein dyes. X. Light absorption and chromophore of litmus". Chem. Ber. 92: 751–3.
- ^ E.T. Wolf: Vollständige Übersicht der Elementar-analytischen Untersuchungen organischer Substanzen, S.450-453, veröffentlicht 1846, Verlag E. Anton (Germany)
