Magnesium stearate
| |
| Names | |
|---|---|
| IUPAC name
Magnesium octadecanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.008.320 |
| E number | E572 (acidity regulators, ...) |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
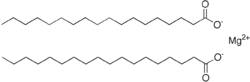
| C36H70MgO4 | |
| Molar mass | 591.27 g/mol |
| Melting point | 88 °C (190 °F; 361 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Magnesium stearate, also called octadecanoic acid, magnesium salt, is a white substance which is solid at room temperature. It has the chemical formula Mg(C18H35O2)2. It is a salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg2+). Magnesium stearate melts at about 88 °C, is not soluble in water, and is generally considered safe for human consumption. Because it is widely regarded as harmless, it is often used as a diluent[1] in the manufacture of medical tablets, capsules and powders.[2] In this regard, the substance is also useful because it has lubricating properties, preventing ingredients from sticking to manufacturing equipment during the compression of chemical powders into solid tablets; magnesium stearate is the most commonly used lubricant for tablets.[3] It is used to bind sugar in hard candies. It is also a common ingredient in baby formulas. In pure powder form, the substance can be a dust explosion hazard,[4] although this issue is effectively insignificant beyond the manufacturing plants using it.
When used as a filling agent in the manufacture of capsules and tablets, such as vitamins, the source of this ingredient is typically bovine.[citation needed] However, there is an increasing number of vegetarian options in which the product specifically indicates it contains magnesium stearate from vegetable sources.
Magnesium stearate is a major component of "bathtub rings". When produced by soap and hard water, magnesium stearate and calcium stearate both form a white solid insoluble in water, and are collectively known as "soap scum".
References
- ^ http://pubs.acs.org/cen/whatstuff/86/8601sci3.html
- ^
Sworbrick, James (1990). Encyclopedia of pharmaceutical technology. p. 2274. ISBN 0824728246, 9780824728243.
{{cite book}}: Check|isbn=value: invalid character (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^
Weiner, Myra L. (1999). Excipient Toxicity and Safety. p. 10. ISBN 0824782100, 9780824782108.
{{cite book}}: Check|isbn=value: invalid character (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ International Chemical Safety Card 1403
