Mesityl oxide
Appearance

| |

| |
| |
| Names | |
|---|---|
| IUPAC name
4-methylpent-3-en-2-one
| |
| Other names
Mesityl oxide
Isobutenyl methyl ketone Methyl isobutenyl ketone Isopropylidene acetone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.005.002 |
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H10O | |
| Molar mass | 98.145 g·mol−1 |
| Appearance | Oily, colorless to light-yellow liquid[1] |
| Odor | peppermint- or honey-like[1] |
| Density | 0.858 g/cm3 |
| Melting point | −53 °C (−63 °F; 220 K) |
| Boiling point | 129.5 °C (265.1 °F; 402.6 K) |
| 3% (20°C)[1] | |
| Solubility in other solvents | Soluble in most organic solvents |
| Vapor pressure | 9 mmHg (20°C)[1] |
Refractive index (nD)
|
1.442 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
flammable |
| Flash point | 31 °C; 87 °F; 304 K [1] |
| Explosive limits | 1.4%-7.2%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1120 mg/kg (rat, oral) 1000 mg/kg (rabbit, oral) 710 mg/kg (mouse, oral)[2] |
LC50 (median concentration)
|
1000 mg/m3 (rat, 4 hr) 9000 mg/m3 (rat, 4 hr) 10,000 mg/m3 (mouse, 2 hr) 2000 mg/m3 (guinea pig, 7 hr)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 25 ppm (100 mg/m3)[1] |
REL (Recommended)
|
TWA 10 ppm (40 mg/m3)[1] |
IDLH (Immediate danger)
|
1400 ppm[1] |
| Related compounds | |
Related compounds
|
diacetone alcohol acetone, benzylideneacetone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mesityl oxide is a α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a strong cat urine odor.[3]
Synthesis
It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound.[4]
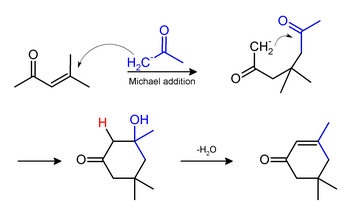
Isophorone may be formed under the same conditions of mesityl oxide production, by a Michael addition. The yields of mesityl oxide and isophorone may vary according to reaction conditions during synthesis:
Uses
Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation:
References
- ^ a b c d e f g h i NIOSH Pocket Guide to Chemical Hazards. "#0385". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Mesityl oxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Merck Index, 11th Edition, 5811
- ^ J. B. Conant and Neal Tuttle (1941). "Mesityl oxide". Organic Syntheses; Collected Volumes, vol. 1, p. 345.