Bromotoluene
Bromotoluenes are aryl bromides based on toluene in which at least one aromatic hydrogen atom is replaced with a bromine atom. They have the general formula C7H8–nBrn, where n = 1–5 is the number of bromine atoms.
Monobromotoluene
[edit]Monobromotoluenes are bromotoluenes containing one bromine atom. There are three isomers, each with the formula C7H7Br.
Properties
[edit]The isomers differ in the location of the bromine, but have the same chemical formula.
| Monobromotoluene isomers[1][2][3] | ||||
|---|---|---|---|---|
| Common name | o-bromotoluene | m-bromotoluene | p-bromotoluene | |
| Structure | 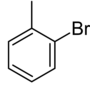
|
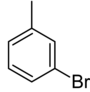
|

| |
| Systematic name | 1-bromo-2-methylbenzene | 1-bromo-3-methylbenzene | 1-bromo-4-methylbenzene | |
| Molecular formula | C7H7Br (C6H4BrCH3) | |||
| Molar mass | 171.03 g/mol | |||
| Appearance | colorless liquid | colorless liquid | white crystalline solid | |
| CAS number | [95-46-5] | [591-17-3] | [106-38-7] | |
| Properties | ||||
| Density and phase | 1.431 g/ml, liquid | 1.4099 g/ml, liquid | 1.3995 g/ml, solid | |
| Solubility in water | practically insoluble | |||
| Other solubilities | very soluble in ethanol, ether, benzene, carbon tetrachloride, acetone, chloroform | |||
| Melting point | -27.8 °C (-18.0 °F; -409.63 K) | -39.8 °C (-39.6 °F; -388.03 K) | 28.5 °C (83.3 °F; 301.7 K) | |
| Boiling point | 181.7 °C (359.1 °F; 454.9 K) | 183.7 °C (362.7 °F; 456.9 K) | 184.5 °C (364.1 °F; 457.7 K) | |
Benzyl bromide is an isomer, which has a bromine substituted for one of the hydrogens of toluene's methyl group, and it is sometimes named α-bromotoluene.
Preparation
[edit]A laboratory route to p-bromotoluene proceeds from p-toluidine, which is diazotiized followed by treatment with copper(I) bromide.[4]
Uses
[edit]Bromotoluenes are precursors to many organic building blocks. For example, the methyl group may be oxidized using potassium permanganate to form the corresponding bromobenzoic acid.[5] The methyl group may also be partially oxidized to form bromobenzaldehyde.[6]
See also
[edit]References
[edit]- ^ "2-Bromotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- ^ "3-Bromotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- ^ "4-Bromotoluene". PubChem. National Center for Biotechnology Information. Retrieved January 19, 2023.
- ^ Bigelow, L. A. (1925). "p-BROMOTOLUENE". Organic Syntheses. 5: 21. doi:10.15227/orgsyn.005.0021.
- ^ Bigelow, L. A. (1922). "A Study of Side-Chain Oxidations with Potassium Permanganate. Ii". Journal of the American Chemical Society. 44 (9): 2010–2019. doi:10.1021/ja01430a020.
- ^ Coleman, G. H.; Honeywell, G. E. (1937). "p-BROMOBENZALDEHYDE". Organic Syntheses. 17: 20. doi:10.15227/orgsyn.017.0020.
